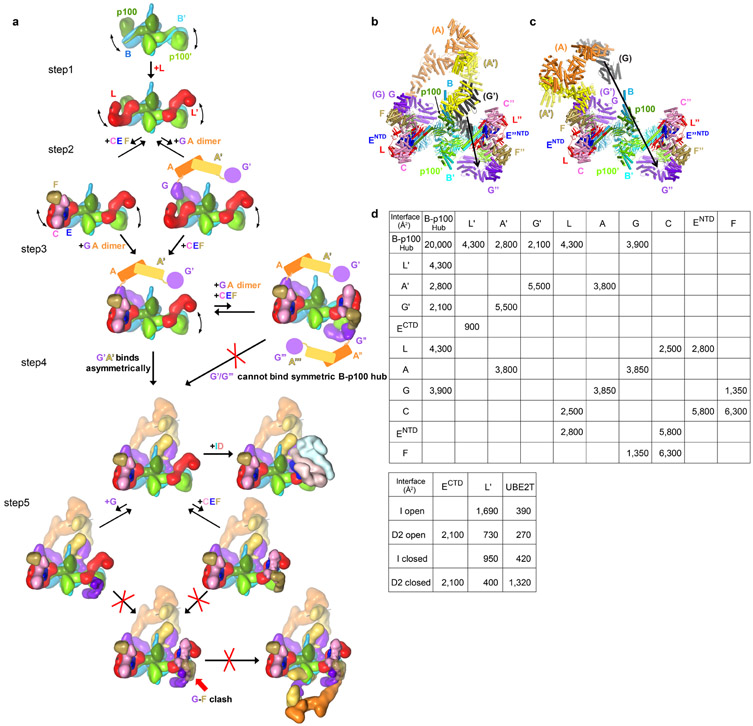
Extended Data Fig. 5. Hypothetical assembly sequence for the Core complex.
a, Individual Core subunits are rendered as low-resolution surfaces and colored as in Figure 1a. At step 1, two FANCL subunits bind to the hub of a dimer of FANCB-FAAP100 heterodimers. At step 2, FANCC-FANCE-FANCF and a dimer of FANCG-FANCA heterodimers transiently bind to the hub, but do not remain stably bound. At step 3, avidity from an interaction between the FANCG and FANCF subunits of the transiently-bound FANCC-FANCE-FANCF and FANCG-FANCA results in their stable association with the hub. This assembly can bind a second FANCG-FANCA dimer of heterodimers and give rise to a fully symmetric but inactive complex that is found in ~1% of the particles (Extended Data Fig. 7). This symmetric complex is rare because FANCA’-FANCG’ association with the hub in step 4 is intramolecular and presumably faster. The Core could, in principle, transiently bind to additional FANCC-FANCE-FANCF and FANCG-FANCA in step 5, but the FANCF-FANCG bridge that would stabilize their transient association with the hub cannot form owing to the different positions of the FANCB’-FAAP100’ WD40 domains and associated FANCL’ induced by FANCG’ binding.
b-c, The FANCA dimer cannot assemble with the hub in a 2-fold symmetric manner. Model of 2-fold symmetric FANCB-FAAP100-FANCL-FANCC-FANCE-FANCF-FANCG with a dimeric FANCA-FANCG subcomplex superimposed on the inactive side of the model in either the inactive-side conformation (FANCG aligned) (b), or in the active side conformation (FANCG’ aligned) (c). The second FANCG of the superimposed subcomplex is in black. Arrow between equivalent atoms indicates the distance (100 Å for b and 220 Å for c) of this second FANCG from the position of FANCG” in the 2-fold symmetric model.
d, Summary of total surface area buried at subunit-subunit interfaces of the apo-Core and the open and closed ID conformation Core-UBE2T-ID.