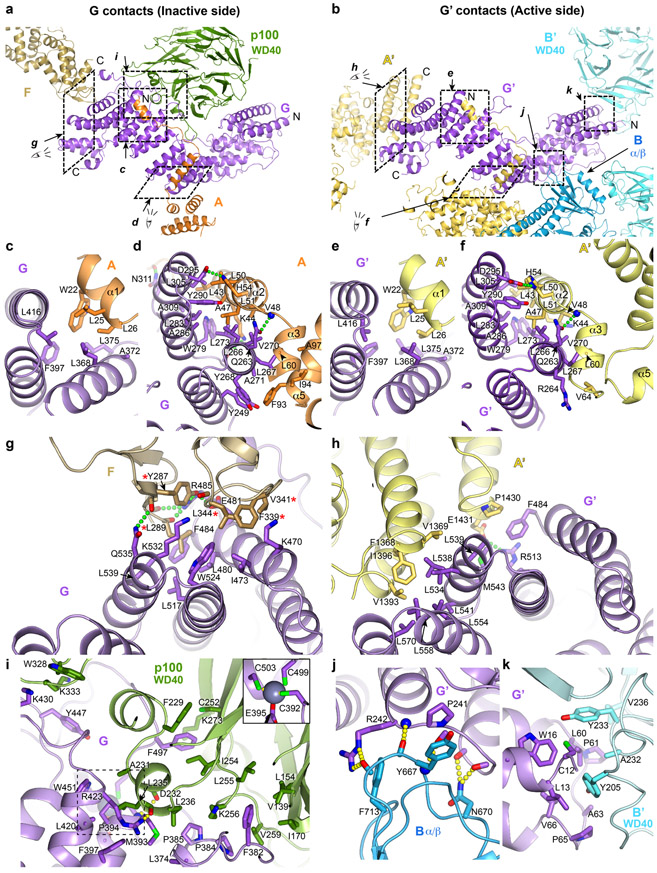
Extended Data Fig. 6. Inter-subunit interactions of FANCG.
a-b, Overall views of FANCG (a) or FANCG’(b), oriented similarly by aligning their central region, also showing their binding partners or portions thereof. Dashed rectangles indicate the regions that the subsequent panels zoom into. The N- and C-termini of FANCG, FANCG’, FANCA’, the C-terminus of FANCF and the N-terminus of FANCA are labeled.
c-f, Close-up views of the contacts between FANCG and to the N-terminal extended segment of FANCA at the inactive-side (c, d) and active-side (e, f) of the apo-Core complex. They are conserved in both sides, except that the FANCA’ α5 helix is uninvolved in contacts due to the different relative orientation of the subsequent FANCA’ NTD with which it packs (Extended Data Fig. 3j).
g, Close-up view of the inactive-side contacts between FANCG and FANCF. Five FANCF residues that when mutated in a cluster compromise Core complex assembly, FANCD2 ubiquitination and ICL resistance31 are marked with a red asterisk.
h, Close-up view of the active-side FANCG’ packing with the C-terminal helical repeats of FANCA’. FANCG’ is oriented as in g to underscore the partial overlap in the FANCF and FANCA’ CTD binding sites of FANCG/FANCG’.
i, Close-up view of the inactive side FANCG binding to the FAAP100 WD40 domain. The inset shows the FANCG zinc-binding site that is otherwise obscured.
j-k, By contrast to the inactive-side FANCG, the active side FANCG’ binds to the FANCB α/β domain (j) and the FANCB’ WD40 domain (k), in two small interfaces. Subunits are colored as in Figure 1a. Only side chains involved in intermolecular interactions are shown. Green or yellow dotted lines indicate hydrogen bond contacts. The interactions are described in Supplementary Note 4.