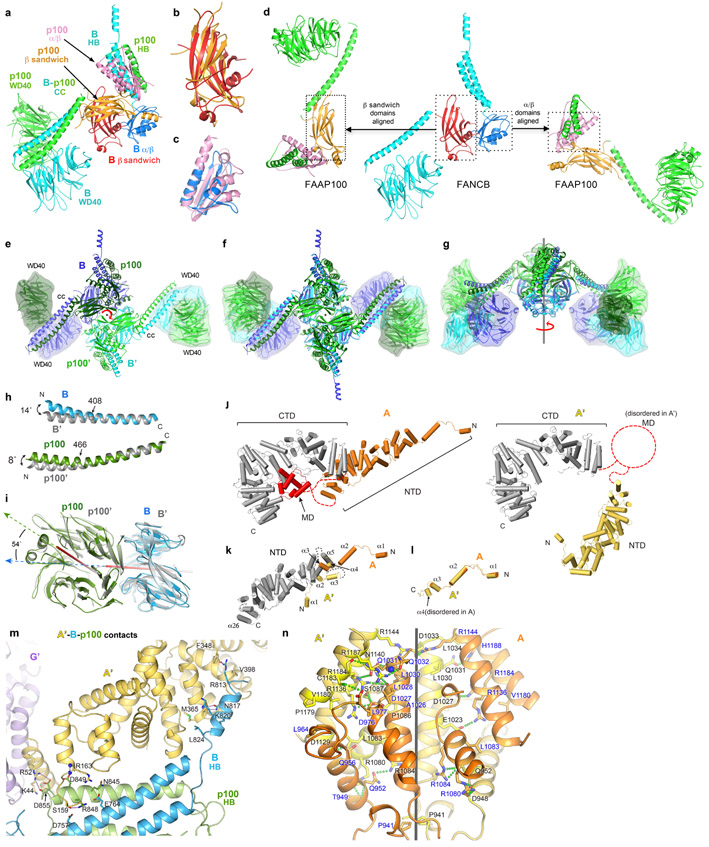
Extended Data Fig. 3. Conformations and dimerization of FANCB-FAAP100 and FANCA.
a, FANCB-FAAP100 heterodimer colored as in Figure 1a, except for the FANCB β sandwich (red) and α/β domain (blue), and the FAAP100 β sandwich (orange) and α/β domain (pink).
b-c, Superposition of the β sandwich (b) and α/β domains (c) of FANCB and FAAP100.
d, Illustration showing the divergent domain-domain packing arrangements of FANCB and FAAP100, aligned within the indicated dotted boxes.
e-g, Dimer of FANCB-FAAP100 heterodimer looking down the 2-fold rotation axis (marked red). WD40 domains are rendered with an envelope to facilitate comparison of their different positions and orientations.
f-g Superposition of the dimer with a 2-fold rotated copy aligned on the central core, looking down the 2-fold axis (f) or rotated 90° about the axis (g), showing the WD40 and most of the coiled-coil domains do not superimpose.
h-i, Superpositions of the coiled-coil helices (h) and the WD40-WD40 pairs (i) at the inactive-side (FANCB blue, FAAP100 green) and active-side (both gray). The helical bend angles and their approximate positions are as shown. Red sticks indicate the internal rotation axis of each WD40 domain; arrows show the directions of the central β strands.
j, Side by side comparison of FANCA (orange NTD) and FANCA’ (yellow NTD), superimposed on their CTD domains (both gray). The MD domain (red) is disordered in FANCA’ (dashed circle).
k-l, Superpositions of the FANCA and FANCA’ NTD domains (gray) (k) and the N-terminal segments (colored as in j) (l).
m, Close-up view of contacts between FANCA’ NTD and the HB domains of FANCB and FAAP100.
n, Close-up view of FANCA dimerization interface, colored as in Figure 1a (black line shows axis of dimer symmetry), showing interacting residues (green-dotted lines indicate hydrogen bonds) (additional discussion of n is in Supplementary Note 2).