Fig. 3. AAV9-mediated delivery of AR45 improves the SBMA phenotype.
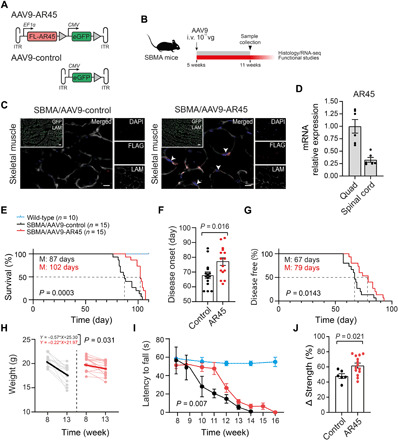
(A) Schematic representation of AAV vectors used in this study. AAV inverted terminal repeats (ITR) and SV40 poly-adenylation signal (triangles) are also indicated. (B) Experimental design; arrow indicates the timing of the intravenous (i.v) injection. Dose is expressed as vector genomes (vg). (C) Representative images of skeletal muscle from SBMA mice treated with AAV9-AR45 or AAV9-control, stained with FLAG antibody (red), LAM (white), GFP (green), and DAPI (blue). Arrowheads indicate AR45 signal. Scale bars, 100 μm (merged) and 10 μm (inset). (D) AR45 mRNA expression levels normalized to GAPDH in spinal cord relative to quadriceps muscle of treated SBMA mice. (E) Kaplan-Meier survival estimation of SBMA mice treated with AAV9-control or AAV9-AR45 (M, median; log-rank test). Survival of wild-type mice is shown for reference. (F and G) AR45-treated SBMA mice show a delay in the disease onset by 12 days in average (M, median; log-rank test) compared to their SBMA littermates. (H) Weight loss is significantly reduced in AR45-treated SBMA mice. Weights for each mouse at the beginning and end of the study are shown in gray (AAV9-control) and pink (AAV9-AR45). (I) Rotarod performances, expressed as latency to fall, are compared between treatment groups [two-way analysis of variance (ANOVA)]. Performances of wild-type mice are also shown for reference. (J) Average percentage reduction in grip strength at the end of the study (week 13) compared to disease onset (week 8) in SBMA mice treated with AAV9-control and AAV9-AR45 is displayed. Data in (D), (F), (I), and (J) are means ± SEM. Each dot represents one replicate.
