Fig. 6. Validation of clinical significance of predicted tumor dependencies by clinical data (TCGA) and preclinical drug trials (PDX Encyclopedia).
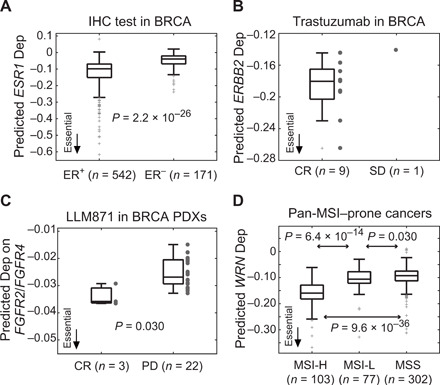
(A) Predicted ESR1 dependencies in breast tumors (BRCA) with positive and negative immunohistochemical (IHC) status of estrogen receptor (ER+ and ER−). Statistical significance is assessed by the one-tailed t test. (B) ERBB2 dependencies in BRCA with different responses to trastuzumab. SD, stable disease. (C) Validation of predicted FGFR2/FGFR4 dependencies by drug response of LLM871 in preclinical PDX models. Predicted dependency scores of FGFR2 and FGFR4 were averaged for each PDX. Statistical significance is assessed by the Kruskal-Wallis test. (D) Predicted WRN dependencies across five MSI-prone cancers of TCGA, including colon adenocarcinoma, esophageal carcinoma, rectum adenocarcinoma, stomach adenocarcinoma, and uterine corpus endometrioid carcinoma. MSI categories were experimentally determined by TCGA: MSI-high (MSI-H), MSI-low (MSI-L), and microsatellite stable (MSS). Statistical significance is assessed by the one-tailed t test. See fig. S15 for the results of individual cancer types and pan-cancer MSI scores predicted by the MANTIS algorithm.
