Fig. 7. Spectroscopic analyses in solution support structural dynamics as a consequence of O2 binding.
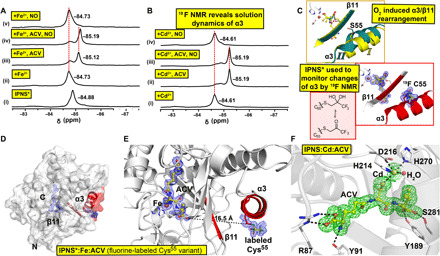
(A) 19F NMR spectra from anaerobic solutions of IPNS* ± Fe2+ ± ACV ± NO support α3 dynamics after NO binding. (i) IPNS* (19F-labeled IPNS); (ii) IPNS* + Fe2+; (iii) IPNS* + Fe2++ACV; (iv) IPNS* + Fe2+ACV + NO; and (v) IPNS* + Fe2++NO. (B) 19F NMR of IPNS* ± Cd2+ ± ACV ± NO. (i) IPNS* + Cd2+; (ii) IPNS* + Cd2++ACV; (iii) IPNS* + Cd2+ACV + NO; and (iv) IPNS* + Cd2++NO (fig. S8). (C) Comparison of the structural elements of α3/β11 in IPNS:Fe:ACV:O2 (PDB: 6ZAP) and IPNS*:Fe:ACV (cryo-cooled single-crystal analysis, PDB: 6ZAM, 1.55-Å resolution, used for 19F NMR, showing keto and gem diol forms of fluorine label). (D) Overview of the IPNS* complex with α3 and β11 in red and presence of the fluorine label on α3. (E) View of the active site and α3 of the IPNS*:Fe:ACV complex, showing the distance from the ACV valinyl carboxylate to the alkylated Cys55 (16.5 Å). Changes in the ACV valinyl carboxylate conformation as a function of O2 binding are proposed to induce structural rearrangement of α3/β11. Cys55 was alkylated with a CH2COCF3 group to study conformational changes in solution by 19F NMR. Tryptic digestion confirmed the presence of alkylated Cys55 (fig. S10). (F) Views of the active site of the aerobically grown IPNS:Cd:ACV complex (PDB: 6ZW8, 1.22-Å resolution) obtained by single-crystal cryogenic data collection. A Polder omit map of the active site of IPNS:Cd:ACV after omitting Cd and ACV (3.0 σ contour level, green, modeled occupancy of 90% for Cd). Unmodeled electron density was observed next to Trp213 on the protein surface. Note that exposure of IPNS:Cd:ACV crystals to NO reveals no NO binding to the metal and no evidence for α3/β11 rearrangements, even after multiple NO exposures with 10 crystals, consistent with the NMR analysis (B).
