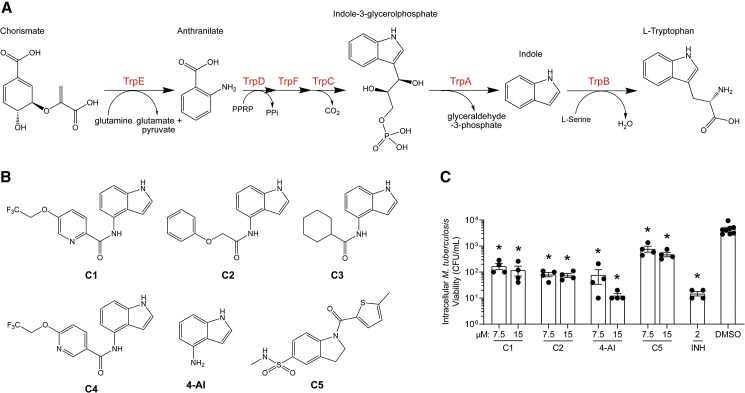
Figure 1.
Antimycobacterial indole-4-carboxamides
(A) The tryptophan biosynthesis pathway and the enzymes (in red) catalyzing each transformation.
(B) Structures of the compounds used in this study.
(C) J774 macrophages were infected with Mtb H37Rv (multiplicity of infection = 1:10) and treated with the corresponding compound at the indicated concentrations for 7 days. The macrophages were lysed and surviving Mtb were enumerated by plating multiple dilutions in 7H11 plates. Limit of detection = 10 CFU/mL. Bars represent mean ± SEM (n = 4). ∗p < 0.001 using one-way ANOVA.