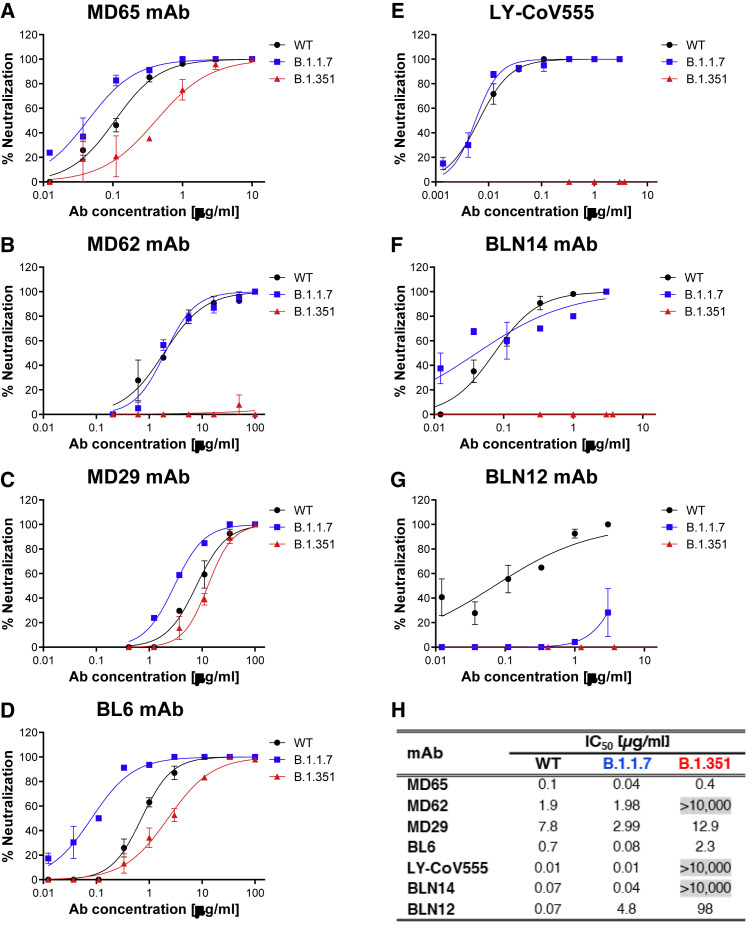
Figure 4.
Neutralization of SARS-CoV-2 B.1.1.7 and B.1.351 by RBD and NTD-specific mAbs
(A–G) Neutralization capacity of the RBD-specific mAbs, MD65 (A), MD62 (B), MD29 (C), BL6 (D), and LY-CoV555 (E) and of the NTD-specific BLN14 (F) and BLN12 (G) was evaluated by plaque reduction neutralization test (PRNT). The in vitro neutralization of each of the listed mAbs was assessed against both SARS-CoV-2 B.1.1.7 (blue) and B.1.351 (red) variant, compared with WT SARS-CoV-2 strain (black). Neutralization potency was determined by the ability of each antibody (at indicated concentrations) to reduce plaque formation. Results are expressed as percent inhibition of control without Ab. Values along the curve depict averages of triplicates ± SEM. The figure includes a representative graph of at least two independent repeats of each experiment, yielding similar results.
(H) Summary of the calculated IC50 values (μg/ml). IC50 > 10,000 indicates complete loss of neutralization capacity, emphasized by gray shading.
The neutralization results, together with previously published biochemical data of the six inspected mAbs, are summarized in Table S1.