Abstract
Dementia and NAFLD are two frequent conditions that share underlying risk factors mainly in the realm of metabolic disease. Additionally, an association between NAFLD and brain aging has been proposed. Therefore, we investigated the hypothesis if NAFLD is an independent risk factor for emerging dementia. In this population-based cohort study, elderly patients (≥ 65 years) with NAFLD diagnosed between 2000 and 2015 were matched 1:1 to a cohort without NAFLD based on ICD-10 coding in the Disease Analyzer database. Matching criteria were age, sex, physician, index year, and co-diagnoses associated with dementia. The primary outcomes of this study were all-cause dementia diagnoses, the incidence of vascular dementia, and antidementive drug prescription. A total of 22,317 patients with NAFLD were matched to 22,317 patients without NAFLD. Within 10 years of the index date, 16.0% of patients with NAFLD and 15.6% of the patients without NAFLD were diagnosed with dementia. On Cox regression analysis, there is no association between NAFLD and the incidence of all-cause dementia (HR 0.97, 95% CI 0.92–1.04), vascular dementia (HR 0.89, 95% CI 0.78–1.02), or the new prescription of antidementive therapy (HR 0.87, 95% CI 0.76–1.01). In sensitivity analyses, there was no association between NAFLD and dementia in different age-groups as well as men or women. In conclusion, in this database study of elderly patients coded with NAFLD no independent association with incident dementia was detected. Risk assessment regarding dementia in patients with NAFLD should be carried out in the same way as for metabolic burdened patients.
Keywords: Cognitive decline, Cognitive impairment, Aging, Metabolic inflammation, Database research study
Introduction
Nonalcoholic fatty liver disease (NAFLD) is the most common liver disease worldwide and has an estimated prevalence of 24% [1–3]. NAFLD is a continuum of different disease stages ranging from liver steatosis (nonalcoholic fatty liver, NAFL) to liver inflammation/hepatitis (nonalcoholic steatohepatitis, NASH) and liver cirrhosis [4]. The mortality risk increases with progressive disease, while fibrosis is the most reliable predictor for higher mortality [5]. In recent years, several studies have demonstrated that NAFLD is not only associated with, for example, impairment in quality of life but also with an increased risk for the development of several other diseases [6–8]. The independent aspect of metabolic liver disease that can be present alongside other liver diseases—including social alcohol use—is currently at the center of an ongoing academic debate [9]. From a pathophysiological point of view, the most obvious independent contribution of NAFLD is with regards to cardiovascular diseases [10–12]. Those findings are in parts explained by an increase in systemic inflammation caused by inflammatory processes in the liver. A recent preclinical study in mice indicated that liver inflammation caused by NAFLD may lead to an activation of microglial cells in the brain and may induce neuronal apoptosis, which results in signs of Alzheimer’s disease (AD) [13]. Another study investigating participants of the Framingham study demonstrated that NAFLD was associated with a smaller total cerebral brain volume pointing to a possible link between NAFLD and earlier brain aging [14]. Additionally, there seems to be a link between advanced stages of NAFLD and poorer cognitive function [15]. However, a potential association between NAFLD and the occurrence of dementia has not been studied yet. Due to the aforementioned evidence and the lack of appropriate data for the primary and secondary care setting in Germany, we investigated the hypothesis that NAFLD may increase the risk for dementia in a population-based cohort study design.
Materials and Methods
Database
This study was conducted using anonymized data derived from the Disease Analyzer database (IQVIA). This database compiles drug prescriptions, diagnoses, and basic medical and demographic data obtained directly and in anonymous format from computer systems used in the practices of general practitioners and specialists [16]. The database covers approximately 3% of all outpatient practices in Germany. Diagnoses [according to International Classification of Diseases, 10th revision (ICD-10)], prescriptions [according to Anatomical Therapeutic Chemical (ATC) Classification system], and the quality of reported data are being monitored by IQVIA. In Germany, the sampling methods used to select physicians’ practices are appropriate for obtaining a representative database of general and specialized practices [16].
Study Population
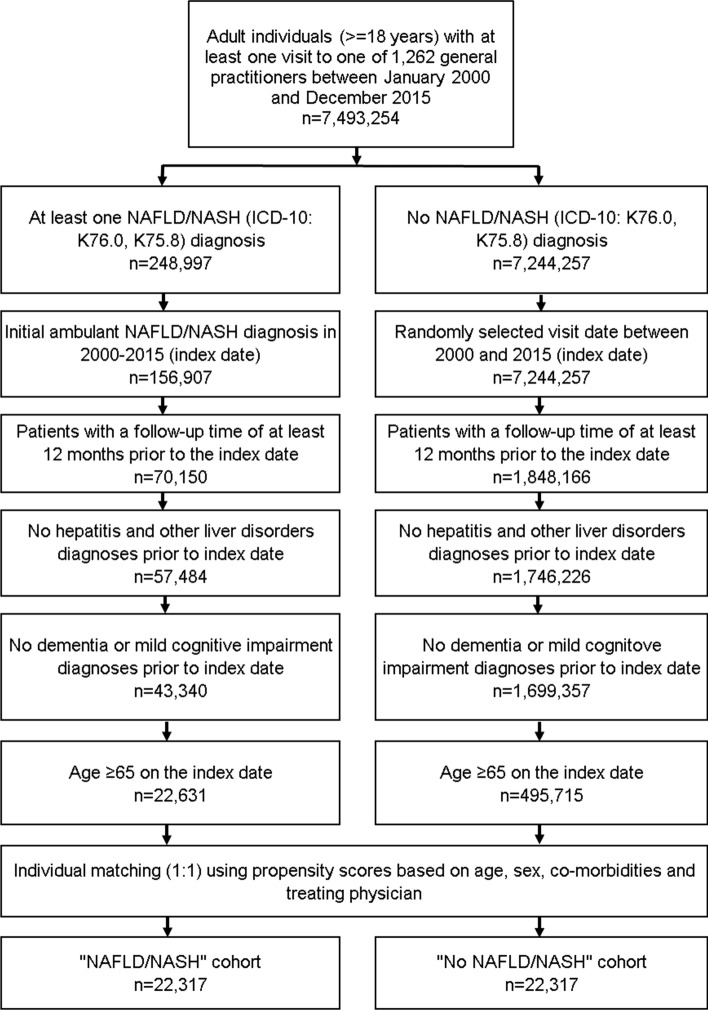
This retrospective cohort study included elderly patients (≥ 65 years) with an initial ICD-10 code of NAFLD/NASH (ICD-10: K75.8, K76.0) in 1262 general practices in Germany between January 2000 and December 2015 (index date; Fig. 1). Further inclusion criterion was an observation time of at least 12 months prior to the index date. Patients with dementia (ICD-10: F00-F03, G30) or mild cognitive impairment diagnoses (ICD-10: F06.7) prior to index date were excluded.
Fig. 1.
Selection of study patients
NAFLD/NASH patients were individually matched to non-NAFLD/NASH patients by age, sex, physician, index year, and metabolic co-diagnoses associated with dementia based on ICD-10 codes including diabetes mellitus (E10-14), hyperlipidemia (E78), ischemic heart disease (I20–I25), arterial hypertension (I10), heart failure (I50), renal failure (N18, N19), stroke and transient ischemic attack (TIA) (I60–I64, G45), intracranial injury (S06), epilepsy (G40, G41), Parkinson’s disease (G20, G21), osteoporosis (M80, M81), obesity (E66), and depression (F32, F33). For the controls, the index date was that of a randomly selected visit between January 2000 and December 2015 (Fig. 1).
Study Outcomes and Covariates
The main outcome of the study was the incidence of all-cause dementia diagnoses as a function of NAFLD/NASH. Furthermore, the incidence of vascular dementia (ICD-10: F01) as well as antidementive drug prescription (ATC: N07D) was assessed.
Statistical Analyses
Differences in the sample characteristics between those with and those without NAFLD/NASH were tested using chi-squared tests for categorical variables and Wilcoxon tests for continuous variables. Cumulative 10-year incidence of all-cause dementia, vascular dementia, and antidementive drugs was calculated using Kaplan–Meier method. Cox regression models were conducted to study the association between the NAFLD/NASH and dementia incidence, vascular dementia incidence, and antidementive drug prescription. These models were performed separately for four age-groups, women and men. p values < 0.05 were considered statistically significant. Analyses were carried out using SAS version 9.4.
Results
Basic Characteristics of the Study Sample
The present study included 22,598 patients with NAFLD/NASH and 22,598 patients without NAFLD/NASH. The basic characteristics of study patients are displayed in Table 1. Mean age (SD) was 73.4 (5.9) years; 53.7% were women. After matching, there was no difference in the patient proportions diagnosed with relevant risk factors for dementia like diabetes, hypertension, hyperlipidemia, ischemic heart disease, heart failure, renal failure, stroke including transitory ischemic attacks, obesity, intracranial injury, epilepsy, Parkinson’s disease, osteoporosis, and depression. The average follow-up time was 7.8 years in patients with NAFLD/NASH and 7.7 years in individuals without NAFLD/NASH, without significant difference.
Table 1.
Basic characteristics of the study sample (after 1:1 matching by age, sex, physician, index year, and CCI)
| Variable | Proportion affected among patients with NAFLD/NASH (%) N = 22,317 |
Proportion affected among patients without NAFLD/NASH (%) N = 22,317 |
p value |
|---|---|---|---|
| Age (mean, SD) | 73.4 (5.9) | 73.4 (5.9) | 1.000 |
| Age 65–70 | 37.6 | 37.6 | 1.000 |
| Age 71–75 | 29.1 | 29.1 | |
| Age 75–80 | 20.3 | 20.3 | |
| Age > 80 | 13.0 | 13.0 | |
| Women | 53.7 | 53.7 | 1.000 |
| Men | 46.3 | 46.3 | |
| Comorbidities at baseline | |||
| Diabetes | 42.1 | 42.1 | 1.000 |
| Hypertension | 72.4 | 72.4 | 1.000 |
| Hyperlipidemia | 56.7 | 56.7 | 1.000 |
| Ischemic heart disease | 31.4 | 31.4 | 1.000 |
| Heart failure | 13.3 | 13.3 | 1.000 |
| Renal failure | 12.2 | 12.2 | 1.000 |
| Stroke including TIA | 16.5 | 16.5 | 1.000 |
| Obesity | 17.8 | 17.8 | 1.000 |
| Intracranial injury | 0.3 | 0.3 | 1.000 |
| Epilepsy | 0.7 | 0.7 | 1.000 |
| Parkinson’s disease | 1.0 | 1.0 | 1.000 |
| Osteoporosis | 9.0 | 9.0 | 1.000 |
| Depression | 14.7 | 14.7 | 1.000 |
Proportions of patients in % given, unless otherwise indicated
SD standard deviation
Association of NAFLD/NASH and Incidence of Dementia
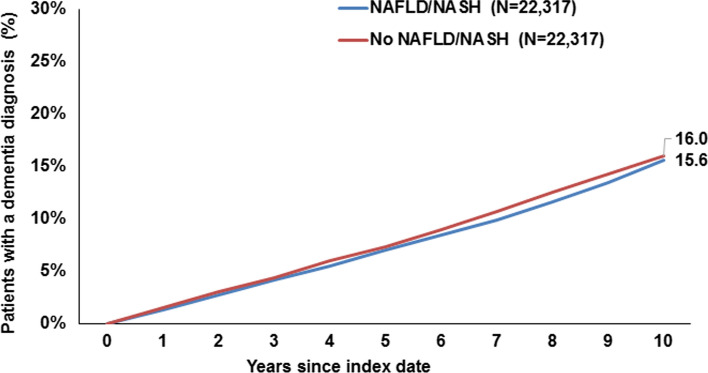
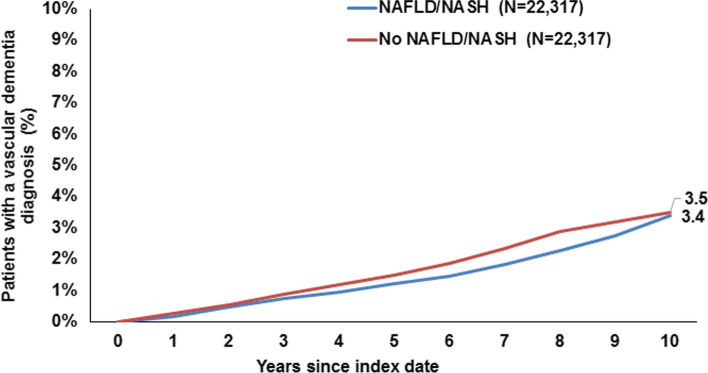
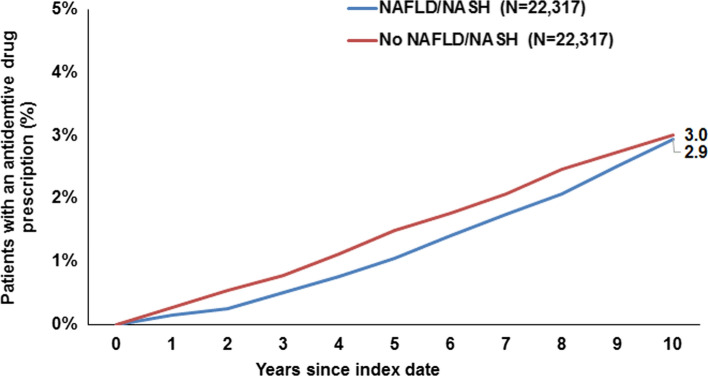
Ten years from the index date, 16.0% of patients with NAFLD/NASH and 15.6% of individuals without NAFLD/NASH were diagnosed with dementia overall, and this difference was not significant (Fig. 2). There were no significant differences in the cumulative incidence of vascular dementia only (3.4% vs. 3.5%, Fig. 3) or antidementive drug prescription (2.9% vs. 3.0%, Fig. 4). On regression analyses, NAFLD/NASH was not associated with the incidence of all-cause dementia, vascular dementia, or antidementive therapy. There was no significant association in four age-groups and men and women (Table 2).
Fig. 2.
Cumulative incidence of all-cause dementia diagnosis in patients with and without NAFLD/NASH
Fig. 3.
Cumulative incidence of vascular dementia diagnosis in patients with and without NAFLD/NASH
Fig. 4.
Cumulative incidence of antidementive drug prescription in patients with and without NAFLD/NASH
Table 2.
Association between NAFLD/NASH and the incidence of all-cause dementia, vascular dementia, and antidementive therapy in patients followed in general practices in Germany (Cox regression models)
| Variable | All-cause dementia | Vascular dementia | Antidementive therapy | |||
|---|---|---|---|---|---|---|
| HR (95% CI)a | p value | HR (95% CI)a | p value | HR (95% CI)a | p value | |
| Total | 0.97 (0.92–1.04) | 0.403 | 0.89 (0.78–1.02) | 0.102 | 0.87 (0.76–1.01) | 0.059 |
| Age 65–70 | 0.91 (0,79–1.05) | 0.179 | 0.85 (0.63–1.15) | 0.296 | 0.86 (0.81–1.37) | 0.341 |
| Age 71–75 | 0.93 (0.83–1.05) | 0.242 | 0.89 (0.69–1.15) | 0.363 | 1.05 (0.81–1.37) | 0.705 |
| Age 75–80 | 1.04 (0.92–1.17) | 0.537 | 0.81 (0.62–1.04) | 0.101 | 0.80 (0.62–1.04) | 0.091 |
| Age > 80 | 0.95 (0.84–1.07) | 0.372 | 1.01 (0.76–1.35) | 0.934 | 0.72 (0.62–1.08) | 0.139 |
| Women | 0.99 (0.91–1.07) | 0.791 | 0.84 (0.70–1.01) | 0.067 | 0.91 (0.75–1.10) | 0.319 |
| Men | 0.96 (0.87–1.05) | 0.354 | 0.96 (0.78–1.18) | 0.716 | 0.83 (0.67–1.02) | 0.079 |
aDifferences in the sample characteristics between those with and those without NAFLD/NASH were tested using chi-squared tests for categorical variables and Wilcoxon tests for continuous variables
Discussion
In this large database study comparing NAFLD of all stages with a matched non-NAFLD population, we did not observe an increased risk of being coded with dementia in a population aged 65 years and above. The risk for emerging dementia of all causes was not increased in patients with NAFLD compared to patients without coded NAFLD. This finding is upheld in subgroup analyses including men, women as well as different age-groups. Additionally, no association was observed, when focusing only on vascular dementia as a primary endpoint.
As recently described, the overall coded prevalence for NAFLD in this German database is low with 3.3% and most likely underestimates the true prevalence of NAFLD, which has been estimated to be as high as 25% [17]. This trend for undercoding of NAFLD in a primary care setting was also recently reported in a study, which included data from Italy, the Netherlands, Spain, and the UK [18, 19]. Here, the coded prevalence for NAFLD was only 2%.
Overall, dementia is a major cause of morbidity and mortality in the western world and affects millions of patients worldwide. Recent studies investigating the Framingham study demonstrated that NAFLD was associated with a smaller total cerebral brain volume pointing and poorer cognitive function as determined by magnet resonance imaging [14, 15]. Our current study adds to the existing literature and highlights that in a heterogeneous, elderly groups of patients carrying an ICD-10 code with NAFLD in Germany, the incidence of dementia was not increased. A recent study in the same database observed that ICD-10 codes of NAFLD are not associated with stroke, which is closely linked to vascular dementia [10]. On the other hand, others have shown an association with white matter injury [20] and altered liver enzymes have recently been associated with early Alzheimer’s disease markers [21]. The divergent results are in part explained by differences in the study populations explored and, importantly, the failure to identify patients with an advanced stages or NASH in the presented database analysis. From a translational point of view, metabolic inflammation in various compartments as an underlying cause of cognitive impairment is highly prevalent in these patients [12, 22]. Taken together, these results justify prospective studies to explore a link between dementia and NAFLD.
Within the spectrum of dementive diseases, Alzheimer’s disease is the most common cause of dementia in older individuals and its pathogenesis is characterized by synaptic and neuronal degeneration and amyloid plaques, which are primarily composed of amyloid beta peptide [23]. The liver—along with the kidney—plays a central role in the clearance of peripheral circulating amyloid beta through metabolic detoxification. In the state of hepatic inflammation and especially in more advanced stages like cirrhosis, amyloid beta uptake into hepatocytes and consequently excretion into the bile may be impaired leading to higher amyloid beta levels in the circulation [22, 24–26]. A recent genetic analysis performing network clustering analyses found 189 shared genes between NAFLD and Alzheimer’s disease and illustrated shared pathways between both diseases including long fatty acid metabolism, IL-17 signaling, and carbohydrate metabolism [27]. This leaves the notion that in particular obesity and diabetes are strong risk factors for NAFLD and are also contributing to Alzheimer’s disease. The current analysis underlines the importance of comorbidities—and here in particular metabolic comorbidities—in determining the risk to develop dementia, and importantly there was no independent impact of NAFLD observed in this database. Other aspects include the low risk of dementia in the presence of NAFLD without any metabolic comorbidities, and as such even in this large database, the effect was not detectable. Additionally, clearance of amyloid beta from the circulation may only be impaired in more advanced stages of liver diseases like liver cirrhosis and all patients with end-stage liver disease coded for cirrhosis were excluded. Therefore, the studied cohort is likely a patient sample that is dominated by earlier stages of NAFLD. This may have an important impact on the dementia risk, since fibrosis severity seems to be with markers of brain injury and cognition [15]. Unfortunately, no data related to laboratory results and no surrogate scores for the presence of advanced fibrosis are available to separate patients with advanced fibrosis from those with earlier disease [28]. Future studies should be conducted to replicate or reject these findings and investigate the impact of disease stage on the development of dementia. Additionally, subtle cognitive decline will not be captured in the type of databases explored herein and will have to be assessed in prospective investigations using defined tools.
Our study has several strengths. These include the large dataset that was exploited resulting in over 22,000 patients with NAFLD and matched controls. Due to extensive matching, potential bias caused by other comorbidities beyond NAFLD could be reduced. Furthermore, the German Disease Analyzer database has been used and its reliability has been validated in several medical studies that consequently strengthen the reliability of our findings [16]. However, there are also some limitations that have to be acknowledged. First, the coded prevalence of NAFLD was with 3.3% by far lower than the estimated prevalence of NAFLD in Europe [17]. Therefore, it seems possible that the representativeness of our selected study sample is limited. Our current study relies on ICD-10 codes for established diagnoses and is therefore prone to misclassification bias due to miscoding or undercoding. It seems possible that some participants included into our control group may suffer from undiagnosed/uncoded NAFLD. Moreover, we did not adjust for potential competing risk during the study period, which could introduce additional bias. Lastly, the ICD-10 codes do not allow to capture lifestyle factors associated with dementia, including alcohol consumption, smoking, or physical activity and therefore we are unable to adjust for these confounders.
In conclusion, this database analysis did not observe an independent impact of coded NAFLD on the risk of emerging dementia. Currently, risk assessment regarding dementia in patients with NAFLD should be carried out in the same way as for the general population.
Abbreviation
- NAFLD
Nonalcoholic fatty liver disease
Author’s contribution
CL, KK, and JMS performed the research; CL, LK, PRG, KK, and JMS designed the experiments and analyzed the data; KK contributed to reagents/materials/analysis tools. CL and JMS wrote the paper. KK helped in statistical analysis. All authors approved the final version of the manuscript and the authorship list. JMS is the guarantor of the article.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was not supported by any grant or funding source.
Data availability
Data are available from KK on the discretion of IQVIA.
Compliance with Ethical Standards
Conflict of interest
JMS has received personal fees for consultancy to BMS, Echosens, Genfit, Gilead Sciences, Intercept Pharmaceuticals, Madrigal, Novartis, Pfizer, and Roche. Research funding is obtained from Gilead Sciences that serves on the speakers’ bureau for the Falk Foundation. KK is an employee of IQVIA. The other authors disclose no potential financial or non-financial conflict of interests.
Ethical standards
This study was conducted according to the ethical guidelines of the 1975 Declaration of Helsinki (6th revision, 2008). The study explored anonymous electronic medical records for research purposes with no directly identifiable data. Accordingly, this study did not collect informed consent from individual patients. Anonymized data were analyzed as aggregates with no protected health information available.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi Z, Tacke F, Arrese M, et al. Global perspectives on nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology. 2019;69:2672–2682. doi: 10.1002/hep.30251. [DOI] [PubMed] [Google Scholar]
- 2.Schuppan D, Schattenberg JM. Non-alcoholic steatohepatitis: pathogenesis and novel therapeutic approaches. J Gastroenterol Hepatol. 2013;28:68–76. doi: 10.1111/jgh.12212. [DOI] [PubMed] [Google Scholar]
- 3.Lazarus JV, Colombo M, Cortez-Pinto H, et al. NAFLD—sounding the alarm on a silent epidemic. Nat Rev Gastroenterol Hepatol. 2020;17:377–379. doi: 10.1038/s41575-020-0315-7. [DOI] [PubMed] [Google Scholar]
- 4.Schattenberg JM, Schuppan D. Nonalcoholic steatohepatitis: the therapeutic challenge of a global epidemic. Curr Opin Lipidol. 2011;22:479–488. doi: 10.1097/MOL.0b013e32834c7cfc. [DOI] [PubMed] [Google Scholar]
- 5.Taylor RS, Taylor RJ, Bayliss S, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625.e1612. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 6.Huber Y, Boyle M, Hallsworth K, et al. Health-related quality of life in nonalcoholic fatty liver disease associates with hepatic inflammation. Clin Gastroenterol Hepatol. 2019;17:2085–2092.e2081. doi: 10.1016/j.cgh.2018.12.016. [DOI] [PubMed] [Google Scholar]
- 7.Kaps L, Labenz C, Galle PR, Weinmann-Menke J, Kostev K, Schattenberg JM. Non-alcoholic fatty liver disease increases the risk of incident chronic kidney disease. United Eur Gastroenterol J. 2020;8:942–948. doi: 10.1177/2050640620944098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maher JJ, Schattenberg JM. Nonalcoholic fatty liver disease in 2020. Gastroenterology. 2020;158:1849–1850. doi: 10.1053/j.gastro.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eslam M, Newsome PN, Anstee QM, et al. A new definition for metabolic associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. [DOI] [PubMed]
- 10.Labenz C, Huber Y, Michel M, et al. Impact of NAFLD on the incidence of cardiovascular diseases in a primary care population in Germany. Dig Dis Sci. 2019;65:2112–2119. doi: 10.1007/s10620-019-05986-9. [DOI] [PubMed] [Google Scholar]
- 11.Labenz C, Prochaska JH, Huber Y, et al. Cardiovascular risk categories in patients with nonalcoholic fatty liver disease and the role of low-density lipoprotein cholesterol. Hepatol Commun. 2019;3:1472–1481. doi: 10.1002/hep4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azzu V, Vacca M, Virtue S, Allison M, Vidal-Puig A. Adipose tissue-liver cross talk in the control of whole-body metabolism: implications in nonalcoholic fatty liver disease. Gastroenterology. 2020;158:1899–1912. doi: 10.1053/j.gastro.2019.12.054. [DOI] [PubMed] [Google Scholar]
- 13.Kim DG, Krenz A, Toussaint LE, et al. Non-alcoholic fatty liver disease induces signs of Alzheimer’s disease (AD) in wild-type mice and accelerates pathological signs of AD in an AD model. J Neuroinflammation. 2016;13:1. doi: 10.1186/s12974-015-0467-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weinstein G, Zelber-Sagi S, Preis SR, et al. Association of nonalcoholic fatty liver disease with lower brain volume in healthy middle-aged adults in the Framingham study. JAMA Neurol. 2018;75:97–104. doi: 10.1001/jamaneurol.2017.3229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weinstein G, Davis-Plourde K, Himali JJ, Zelber-Sagi S, Beiser AS, Seshadri S. Non-alcoholic fatty liver disease, liver fibrosis score and cognitive function in middle-aged adults: the Framingham study. Liver Int. 2019;39:1713–1721. doi: 10.1111/liv.14161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rathmann W, Bongaerts B, Carius HJ, Kruppert S, Kostev K. Basic characteristics and representativeness of the German Disease Analyzer database. Int J Clin Pharmacol Ther. 2018;56:459–466. doi: 10.5414/CP203320. [DOI] [PubMed] [Google Scholar]
- 17.Estes C, Anstee QM, Arias-Loste MT, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 18.Alexander M, Loomis AK, van der Lei J, et al. Risks and clinical predictors of cirrhosis and hepatocellular carcinoma diagnoses in adults with diagnosed NAFLD: real-world study of 18 million patients in four European cohorts. BMC Med. 2019;17:95. doi: 10.1186/s12916-019-1321-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schattenberg JM, Ekstedt M. Assessing the disease burden of non-alcoholic fatty liver disease in the real world—big data and big numbers. BMC Med. 2019;17:123. doi: 10.1186/s12916-019-1357-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petta S, Tuttolomondo A, Gagliardo C, et al. The presence of white matter lesions is associated with the fibrosis severity of nonalcoholic fatty liver. Disease Med (Baltimore) 2016;95:e3446. doi: 10.1097/MD.0000000000003446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nho K, Kueider-Paisley A, Ahmad S, et al. Association of altered liver enzymes with Alzheimer disease diagnosis, cognition, neuroimaging measures, and cerebrospinal fluid biomarkers. JAMA Netw Open. 2019;2:e197978. doi: 10.1001/jamanetworkopen.2019.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehrke N, Schattenberg JM. Metabolic inflammation—a role for hepatic inflammatory pathways as drivers of comorbidities in nonalcoholic fatty liver disease? Gastroenterology. 2020;158:1929–1947.e1926. doi: 10.1053/j.gastro.2020.02.020. [DOI] [PubMed] [Google Scholar]
- 23.Estrada LD, Ahumada P, Cabrera D, Arab JP. Liver dysfunction as a novel player in Alzheimer’s progression: looking outside the brain. Front Aging Neurosci. 2019;11:174. doi: 10.3389/fnagi.2019.00174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rolle A, Paredes S, Cortinez LI, et al. Dexmedetomidine metabolic clearance is not affected by fat mass in obese patients. Br J Anaesth. 2018;120:969–977. doi: 10.1016/j.bja.2018.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Kanekiyo T, Bu G. The low-density lipoprotein receptor-related protein 1 and amyloid-beta clearance in Alzheimer’s disease. Front Aging Neurosci. 2014;6:93. doi: 10.3389/fnagi.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bu XL, Yao XQ, Jiao SS, et al. A study on the association between infectious burden and Alzheimer’s disease. Eur J Neurol. 2015;22:1519–1525. doi: 10.1111/ene.12477. [DOI] [PubMed] [Google Scholar]
- 27.Karbalaei R, Allahyari M, Rezaei-Tavirani M, Asadzadeh-Aghdaei H, Zali MR. Protein–protein interaction analysis of Alzheimer’s disease and NAFLD based on systems biology methods unhide common ancestor pathways. Gastroenterol Hepatol Bed Bench. 2018;11:27–33. [PMC free article] [PubMed] [Google Scholar]
- 28.Labenz C, Huber Y, Kalliga E, et al. Predictors of advanced fibrosis in non-cirrhotic non-alcoholic fatty liver disease in Germany. Aliment Pharmacol Ther. 2018;48:1109–1116. doi: 10.1111/apt.14976. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available from KK on the discretion of IQVIA.