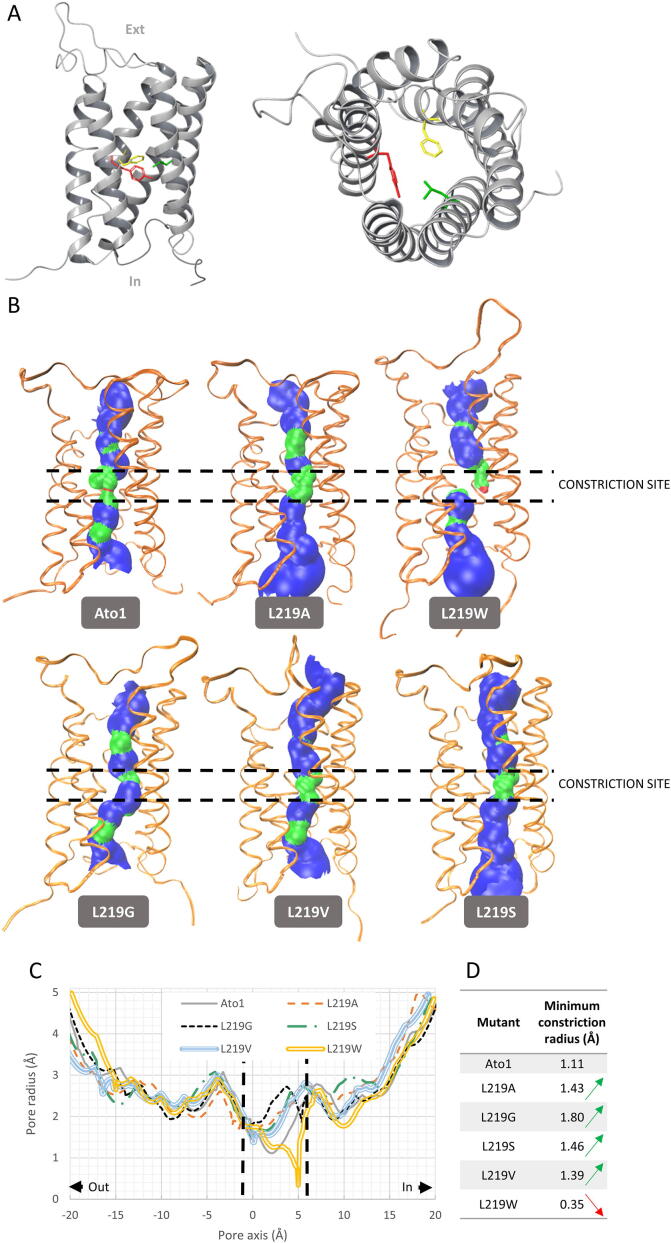
Fig. 3.
Predicted 3D structure model of Ato1 obtained by homology threading using SatP_Ck crystal structure (PDB Entry: 5YS3) as template. A – Ato1 consists of six transmembrane α-helices with both termini at the intracellular side. The residues F98 (yellow), Y155 (red), and L219 (green) in the 3D structure of the Ato1 (transversal and extracellular view) form the central and narrowest hydrophobic constriction of the anion pathway. In-intracellular; Ext-extracellular. B – Analysis of the effect of the mutations L219A, L219G, L219S, L219V and L219W on the protein 3D structure using HOLE software (see Material and Methods). C – Prediction of the pore constriction along the axis of the native Ato1 and of mutations L219A, L219G, L219S, L219V and L219W using HOLE software. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)