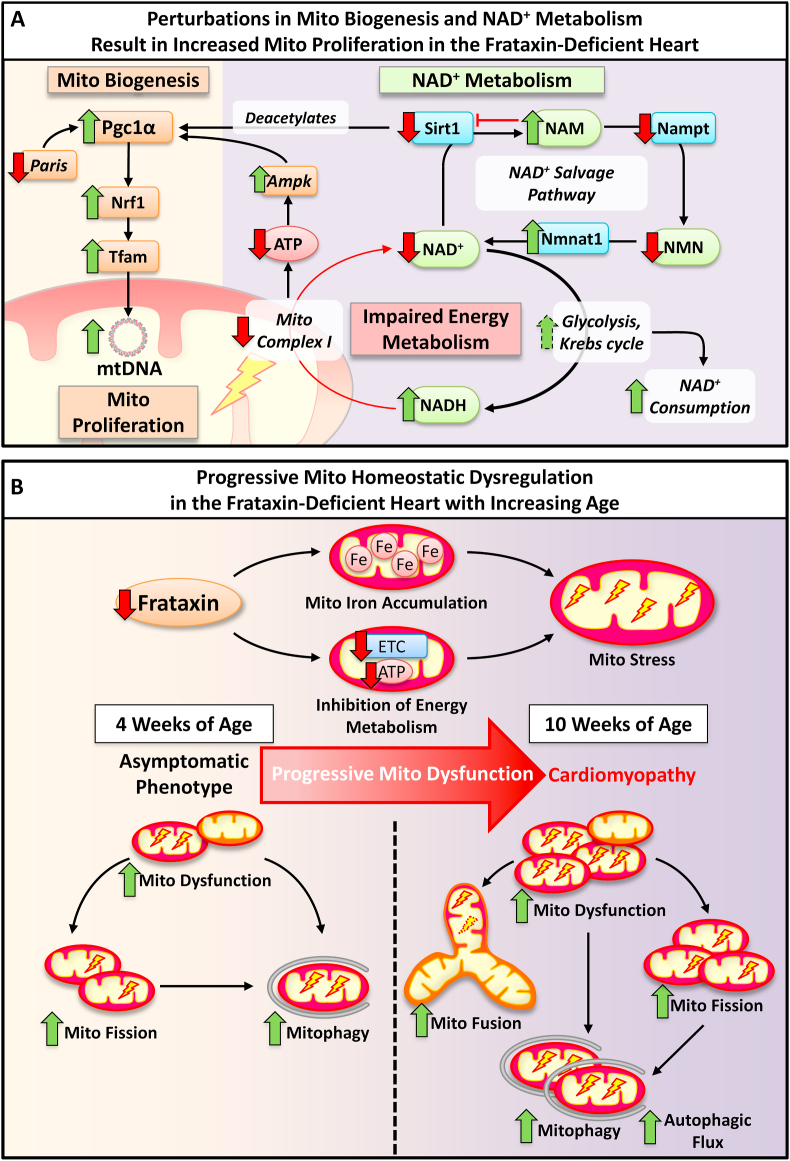
Fig. 8.
Schematic illustrating the effects of cardiac frataxin-deficiency on the development of mitochondrial (Mito) homeostatic dysregulation. (A) In 10 week-old frataxin KO mice hearts, enhanced mitochondrial proliferation was evident by the up-regulation of mitochondrial biogenesis (involving Pgc1α, Nrf1 and Tfam) and increased mtDNA. The increased Pgc1α protein could be potentiated by transcriptional regulation via increased Ampk and decreased Paris. Concurrently, there was also increased Pgc1α acetylation suggesting inhibition of the protein at 10 weeks, which could be due to decreased Sirt1 expression and activity caused by a feedback inhibition due to increased NAM. The NAD+ salvage pathway was also dysregulated with the inhibition of Nampt but up-regulation of Nmnat1, causing increased NAM and reduced NMN, respectively. Moreover, increased NAD+ consumption via increased glycolysis and Kreb cycle, together with decreased mitochondrial complex I activity, would result in a decreased NAD+: NADH ratio and impaired energy metabolism in 10 week KOs. (B) In FA, frataxin-deficiency progressively leads to LV mitochondrial iron-accumulation and inhibition of the electron transport chain that causes mitochondrial redox stress. Asymptomatic 4 week frataxin KO mice progressively develop a fatal, hypertrophic cardiomyopathy by 10 weeks. Our data in the frataxin-deficient heart identified progressive activation of: (1) mitochondrial dysfunction, involving the mitochondrial recruitment of Pink1 and Parkin observed from 4 week; (2) mitochondrial fission, involving mitochondrial translocation of Drp1 and increased Fis1 from 4 week; (3) mitochondrial fusion, involving enhanced Mfn1 and Opa1 expression that was significant at 10 weeks; and (4) mitophagy from 4 week, and increased autophagic flux at 10 week. Overall, there is progressive dysregulation of mitochondrial homeostasis in the frataxin-deficient heart that contributes to FA cardiac pathology.