Abstract
Purpose
Lactate is an established prognosticator in critical care. However, there still is insufficient evidence about its role in predicting outcome in COVID-19. This is of particular concern in older patients who have been mostly affected during the initial surge in 2020.
Methods
This prospective international observation study (The COVIP study) recruited patients aged 70 years or older (ClinicalTrials.gov ID: NCT04321265) admitted to an intensive care unit (ICU) with COVID-19 disease from March 2020 to February 2021. In addition to serial lactate values (arterial blood gas analysis), we recorded several parameters, including SOFA score, ICU procedures, limitation of care, ICU- and 3-month mortality. A lactate concentration ≥ 2.0 mmol/L on the day of ICU admission (baseline) was defined as abnormal. The primary outcome was ICU-mortality. The secondary outcomes 30-day and 3-month mortality.
Results
In total, data from 2860 patients were analyzed. In most patients (68%), serum lactate was lower than 2 mmol/L. Elevated baseline serum lactate was associated with significantly higher ICU- and 3-month mortality (53% vs. 43%, and 71% vs. 57%, respectively, p < 0.001). In the multivariable analysis, the maximum lactate concentration on day 1 was independently associated with ICU mortality (aOR 1.06 95% CI 1.02–1.11; p = 0.007), 30-day mortality (aOR 1.07 95% CI 1.02–1.13; p = 0.005) and 3-month mortality (aOR 1.15 95% CI 1.08–1.24; p < 0.001) after adjustment for age, gender, SOFA score, and frailty. In 826 patients with baseline lactate ≥ 2 mmol/L sufficient data to calculate the difference between maximal levels on days 1 and 2 (∆ serum lactate) were available. A decreasing lactate concentration over time was inversely associated with ICU mortality after multivariate adjustment for SOFA score, age, Clinical Frailty Scale, and gender (aOR 0.60 95% CI 0.42–0.85; p = 0.004).
Conclusion
In critically ill old intensive care patients suffering from COVID-19, lactate and its kinetics are valuable tools for outcome prediction.
Trial registration number: NCT04321265.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13613-021-00911-8.
Introduction
The disease caused by Sars-CoV-2, COVID-19, has dominated daily life in numerous intensive care units (ICU) worldwide, since the beginning of 2020. Respiratory failure with or without shock led to high mortality. In ICU admitted patients, up to 30–50% of the patients did not survive the first month [1–3]. Thus, early and reliable identification of complex disease courses is of pivotal importance in COVID-19 (Additional file 1).
In emergency and critical care medicine, serum lactate and its kinetics are useful parameters for critically ill patients as a marker of severity of illness [4–6]. A significant advantage is that the determination of serum lactate is widely and rapidly available as a point-of-care measurement [7]. Hyperlactatemia is an indicator of physiological stress, and anaerobic metabolism, and a “powerful predictor of mortality” [6]. Basically, lactate can be used for two purposes. It can be used both for risk stratification and to monitor the response to therapy. Elevated lactate is a diagnostic criterion for septic shock following the sepsis-3 consensus. Lactate “clearance” is a target parameter for volume substitution in the absence of major liver dysfunction [8]. Although serum lactate and its kinetics have been applied as an essential diagnostic and target parameter in septic patients for more than 20 years, the evidence remains scarce for patients suffering from pneumonia and ARDS.
Despite this lack of evidence, current guidelines recommend the use of lactate and lactate kinetics in COVID-19 [9]. Until now, the value of serum lactate and its kinetics in predicting a severe course in COVID-19 is unclear. This lack of evidence is especially true in the particularly vulnerable population of very old intensive care unit patients. Yet, this subgroup has been disproportionally affected by the need for ICU admissions and a high mortality [3, 10, 11].
This multicenter study addresses this lack of evidence and investigates the value of serum lactate at admission and its kinetics for outcome prediction in a large prospectively enrolled population of older ICU patients.
Methods
Design and settings
This multicenter study is part of the Very old Intensive care Patients (VIP) project and has been endorsed by the European Society of Intensive Care Medicine (ESICM) (https://www.vipstudy.org). The study was registered at ClinicalTrials.gov (ID: NCT04321265) and adhered to the European Union General Data Privacy Regulation (GDPR) directive. This investigation aimed to understand which factors can predict mortality in elderly COVID-19 patients to help detect these patients early (the COVIP study, COVID-19 in very old intensive care patients). As in the previous VIP studies [3, 12, 13], national coordinators recruited the intensive care units (ICUs), coordinated national and local ethical permissions, and supervised patient recruitment at the national level. Ethical approval was mandatory for study participation. In most countries informed consent was obligatory for inclusion. This study extracted patient data from 151 ICUs from 26 independent countries, including European ICUs, and the Asian, African, and Americas.
Study population
The COVIP study recruited consecutieve patients with proven COVID-19 aged 70 years or older who were admitted to an ICU. The data set was extracted from the COVIP study database on 4th February and contained patients from 19th March 2020 to 4th February 2021. Data collection started at ICU admission. Data about pre-ICU triage were not available. The admission day was defined as day 1, and all consecutive days were numbered sequentially from that date.
Data collection
All centers used a uniform online electronic case report form (eCRF). Only patients with a documented highest serum lactate value on days 1 and 2 were included for this subgroup analysis (above 2 mmol/L). Reporting was possible both in [mg/dL] or [mmol/L], depending on local routine. For ease of comparison, all laboratory values were converted to [mmol/] (1 mg/dL = 0.111 mmol/L). The first arterial blood gas (ABG) analysis, including pO2 [mmHg] and the FiO2 [%], was recorded to calculate the pO2/FiO2-ratio on admission. For the sequential organ failure assessment (SOFA) score on admission, each element was entered and the eCRF calculated the total score. Furthermore, we assessed the need for non-invasive or invasive ventilation with its duration, prone positioning, tracheostomy, vasopressor use and renal replacement therapy. The eCRF also documented any limitation of life-sustaining therapy during the ICU-stay. The frailty level prior to the acute illness and hospital admission was assessed using the Clinical Frailty Scale (CFS) [3, 12, 13]. In addition, the eCRF recorded information about gender, age, length of ICU stay symptom onset, and duration of symptoms before ICU-and hospital admission. Furthermore, the eCRF asked about the presence of preexisting comorbidities.
Lactate and ∆ Lactate
Patients were clustered according to their lactate concentration on ICU admission. The arbitrary cutoff value was an initial lactate concentration ≥ 2.0 mmol/L. A lactate value below 2 mmol/L was defined as within the normal range. ∆ Lactate in the first 24 h was defined as maximum serum lactate at admission minus maximum serum lactate on day 2, divided by lactate at admission multiplied by 100 [4]. A positive value indicates a fall in serum lactate and a negative value signifies rising serum lactate. This has been confirmed in larger cohorts as a valuable and simple tool for outcome prediction [4].
Data storage
The eCRF and database were hosted on a secure server in Aarhus University, Denmark.
Statistical analysis
The primary outcome was ICU mortality, secondary outcomes were 30-day and 3-month mortality. Continuous data points were expressed as median and interquartile range. Differences between independent groups were calculated using the MannWhitney U test. Categorical data are expressed as numbers (percentage). The Chi-square test was applied to calculate differences between groups. Univariate und multivariable logistic regression analyses were performed to assess associations with baseline serum lactate and mortality. We chose the co-variables for the multivariable model (age, SOFA score, CFS and gender) based on clinical experience and previous literature [4, 5]. Marginal predictive means with respective 95% confidence intervals (CI) were calculated. All tests were two-sided, and a p value of < 0.05 was considered statistically significant. Stata 16 was used for all statistical analyses (StataCorp LLC, 4905 Lakeway Drive, College Station, Brownsville, Texas, USA).
Results
Study population
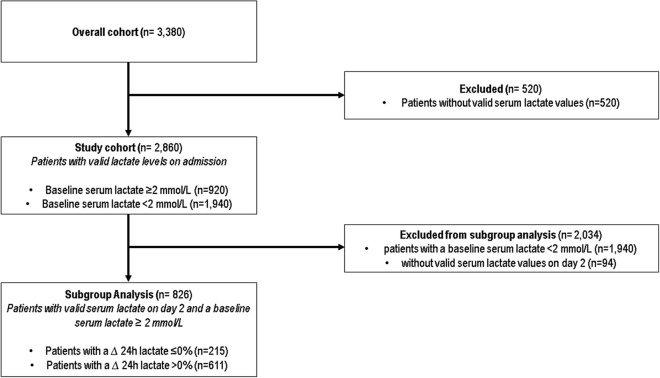
In total, 2860 patients with an available baseline serum lactate value were included (Fig. 1). Overall, 68% (1940 patients) patients had no abnormal elevation of serum lactate on the day of admission to the ICU, while 32% (920 patients) evidenced an elevated serum lactate (Fig. 2) (Table 1).
Fig. 1.
CONSORT diagram
Fig. 2.
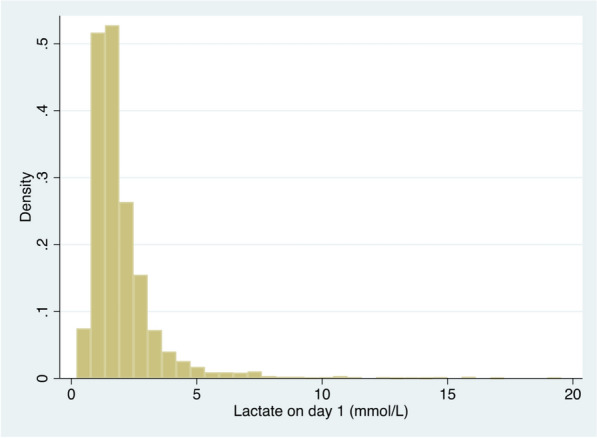
Distribution of maximum serum lactate values on day 1 (= day of ICU-admission), [mmol/L]
Table 1.
Baseline characteristics
| Variables | Baseline lactate ≥ 2 mmol/L | Baseline lactate < 2 mmol/L | p value |
|---|---|---|---|
| n = 920 (32%) | n = 1940 (68%) | ||
| Male gender [n] (%) | 678 (74%) | 1356 (70%) | 0.033 |
| Age [years] (IQR) | 72–79 (76) | 72–78 (75) | 0.007 |
| Age 70–79 [n] (%) | 715 (78%) | 1554 (80%) | 0.15 |
| Age 80–89 [n] (%) | 194 (21%) | 373 (19%) | 0.24 |
| Age > 90 [n] (%) | 10 (1%) | 12 (1%) | 0.18 |
| CFS (IQR) | 2–4 (3) | 2–4 (3) | 0.47 |
| Diabetes [n] (%) | 334 (37%) | 690 (36%) | 0.68 |
| Coronary vascular disease [n] (%) | 226 (25%) | 441 (23%) | 0.26 |
| Chronic renal failure [n] (%) | 163 (18%) | 331 (17%) | 0.65 |
| Arterial hypertension [n] (%) | 599 (65%) | 1325 (69%) | 0.086 |
| Pulmonary disease [n] (%) | 200 (22%) | 456 (24%) | 0.30 |
| Heart failure [n] (%) | 164 (18%) | 264 (14%) | 0.004 |
| Lactate on day 1 [mmol/L] (IQR) | 2.3–3.8 (2.8) | 1.1–1.7 (1.4) | < 0.001 |
| SOFA score (IQR) | 4–8 (6) | 3–8 (5) | < 0.001 |
| Symptoms prior to hospitalization (days) | 2 (1–5) | 2 (1–5) | 0.96 |
| Symptoms prior to ICU-admission (days) | 6 (3–9) | 7 (3–10) | 0.38 |
CFS clinical frailty scale, SOFA score sequential organ failure Assessment for the first 24 h, IQR interquartile range
Baseline serum lactate
Patients with a lactate greater than/equal to 2 mmol/L were older [76 (72–79) vs. 75 (72–78) years, p = 0.007], but not more frail [CFS 3 (2–4) vs. 3 (2–4), p = 0.47]. Patients with elevated serum lactate had a higher SOFA score on admission (Table 1). There were no differences in symptom duration or time in the hospital before ICU admission [Symptoms prior to hospitalization 2 days (1–5) vs. 2 days (1–5), p = 0.96; symptoms prior to ICU-admission 6 days (3–9) vs. 7 days (3–10), p = 0.38]. Both groups evidenced similar incidences of pre-existing comorbidities (diabetes, coronary vascular disease, chronic renal failure, arterial hypertension, pulmonary disease) with the exception for heart failure, which was significantly more often in patients with an elevated baseline serum lactate (18% vs. 14%, p = 0.004).
On day 2, the maximum serum lactate was lower in the group of patients with initially elevated serum lactate than on the day of admission, but still significantly higher than in the group of patients with non-elevated lactate on admission [2.2 mmol/L (1.7–3.0) vs. 1.4 mmol/L (1.1–1.8), p < 0.001]. Patients with an elevated baseline serum lactate had significantly higher ∆ lactate in 24 h than patients with a normal baseline lactate [25% (0–45) vs. − 7% (− 33–12), p < 0.001] (Table 1). The two groups demonstrated differences in intensive care therapy. Patients with an elevated baseline lactate were significantly more likely to receive mechanical ventilation [74% (677) vs. 70% (1359), p = 0.042], renal replacement therapy [19% (171) vs. 15% (288), p = 0.013], and vasoactive drugs [72% (654) vs. 68% (1311), p = 0.038], but prone positioning occurred significantly less often in patients with an elevated serum lactate [49% (329) vs. 57% (764), p < 0.001]. There was no difference regarding non-invasive ventilation [25% (230) vs. 26% (501), p = 0.61] or tracheostomy [19% (173) vs. 19% (364), p = 0.95]. Patients with elevated baseline lactate had a significantly higher ICU-, 30-day and 3-month mortality (Table 2). Length of ICU was lower in patients with an elevated baseline serum lactate [336 h (408) vs. 377 (433), p = 0.037]. After exclusion of non-survivors, there was no difference between both groups [475 h (526) vs. 570 h (552), p = 0.48]. Accordingly, the duration of mechanical ventilation was significantly longer in patients with normal baseline [343 h (360) vs. 367 h (360), p = 0.026]. Again, after exclusion of non-survivors and patients without mechanical ventilation, there remained no significant difference between both groups [323 h (414) vs. 311 (445) h, p = 0.41]. Treatment was withheld in 30% (271) of the patients with an elevated and in 30% (565) of the patients with a normal baseline lactate (p = 0.85). Treatment was withdrawn in 21% (188) and 18% (348), respectively (p = 0.12).
Table 2.
Primary and secondary outcomes
| Variables | Baseline Lactate ≥ 2 mmol/L | Baseline Lactate < 2 mmol/L | p value |
|---|---|---|---|
| n = 920 (32%) | n = 1940 (68%) | ||
| ICU mortality | 465 (53%) | 817 (43%) | < 0.001 |
| 30-day mortality | 496 (56%) | 854 (46%) | < 0.001 |
| 3-month mortality | 533 (71%) | 924 (57%) | < 0.001 |
| ICU length of stay (hours, IQR) | 336 (408) | 377 (433) | 0.037 |
| Duration of mechanical ventilation (hours, IQR) | 343 (360) | 367 (360) | 0.026 |
ICU intensive care unit
In a univariate regression analysis, the baseline lactate was significantly associated with ICU mortality (OR 1.12 95% CI 1.07–1.17; p < 0.001), 30-day mortality (OR 1.11 95% CI 1.06–1.16; p < 0.001) and 3-month mortality (OR 1.16 95% CI 1.09–1.23; p < 0.001).
In the multivariable analysis, the maximum lactate concentration on day 1 was independently associated with ICU mortality (aOR 1.06 95% CI 1.02–1.11; p = 0.007), 30-day mortality (aOR 1.07 95% CI 1.02–1.13; p = 0.005) and 3-month mortality (aOR 1.15 95% CI 1.08–1.24; p < 0.001) after adjustment for age, SOFA, CFS and sex (Fig. 3).
Fig. 3.
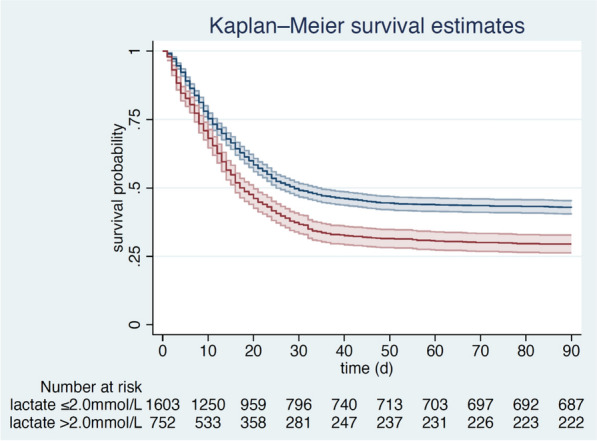
Kaplan–Meier for patients with a baseline lactate ≥ 2 mmol/L (red line) compared to patients with a baseline lactate < 2 mmol/L (blue line) (3-month mortality, ± standard deviation)
∆ Serum lactate in 24 h
In 826 patients (29%), there was a baseline lactate ≥ 2 mmol/L and sufficient data to calculate the ∆ serum lactate over 24 h. In both sub-groups, patients were predominantly male (p = 0.37, Table 3). There were no differences regarding age, SOFA score on admission, prior hospitalizations, or the duration of symptoms before ICU admission. The median CFS did not differ (Table 3). For pre-existing comorbidities, there was no difference between groups except for pulmonary diseases, which were significantly more common in those patients with rising lactate [27% (n = 58) vs. 19% (n = 117), p = 0.017]. Intensive care treatment, especially regarding non-invasive ventilation [27% (57) vs. 24% (146), p = 0.45], vasoactive drugs [70% (151) vs. 74% (444), p = 0.34], and renal replacement therapy [21% (45) vs. 18% (112), p = 0.42] did not differ between both groups. In patients with ∆ serum lactate over 24 h less than 0, intubation occurred in 71% (152) compared to 76% (464) (p = 0.12), prone positioning was used in 48% (72) compared to 49% (226) (p = 0.76). During the ICU stay, 40 patients with a ∆ serum lactate over 24 h less than 0 received a tracheostomy (19%), while this was true for 109 patients from the group of patients with a ∆ serum lactate ≥ 0% (18%, p = 0.79). Therapy limitations or de-escalations did also not differ between groups. Therapy was withheld in 29% (62) of the patients with a negative ∆ serum lactate, and in 30% (185) of the other group (p = 0.68), while treatment was withdrawn in 19% (40) and 22% (134) of the patients, respectively (p = 0.32). Length of stay was longer in patients with a ∆ Lactate 24 h > 0% [285 (439) h vs. 348 h (400), p = 0.029]. After exclusion of non-survivors, there was no significant difference between both groups [475 (526) h vs. 456 h (552), p = 4.77]. The duration of mechanical ventilation was significantly longer in patients with a positive ∆ Lactate 24 h [210 h (390) vs. 277 (340), p = 0.106]. However, after exclusion of non-survivors, the duration of mechanical ventilation was comparable [323 h (414) vs. 307 h (436) p = 0.364]. ICU mortality was significantly higher for patients with rising lactate [61% (n = 131) vs. 50% (n = 303), p = 0.004, Table 4, Fig. 4].
Table 3.
Baseline characteristics of the subgroup analysis according to the Δ Lactate 24 h [%]
| Variables | ∆ Lactate 24 h ≤ 0% | ∆ Lactate 24 h > 0% | p value |
|---|---|---|---|
| n = 215 (26%) | n = 611 (74%) | ||
| Male gender [n] (%) | 152 (71%) | 447 (73%) | 0.37 |
| Age [years] (IQR) | 73–79 (75) | 72–79 (76) | 0.60 |
| Age 70–79 [n] (%) | 180 (80%) | 484 (76%) | 0.28 |
| Age 80–89 [n] (%) | 42 (19%) | 143 (23%) | 0.22 |
| Age > 90 [n] (%) | 3 (1%) | 6 (1%) | 0.63 |
| CFS (IQR) | 2–4 (3) | 2–4 (3) | 0.13 |
| Diabetes [n] (%) | 75 (35%) | 231 (38%) | 0.43 |
| Coronary vascular disease [n] (%) | 46 (22%) | 154 (26%) | 0.23 |
| Chronic renal failure [n] (%) | 43 (20%) | 102 (17%) | 0.27 |
| Arterial hypertension [n] (%) | 140 (65%) | 403 (66%) | 0.84 |
| Pulmonary disease [n] (%) | 58 (27%) | 117 (19%) | 0.017 |
| Heart failure [n] (%) | 42 (20%) | 108 (18%) | 0.85 |
| SOFA score (IQR) | 4–9 (6) | 4–8 (6) | 0.63 |
CFS clinical frailty scale, SOFA score sequential organ failure assessment for the first 24 h, IQR interquartile range
Table 4.
Primary and secondary outcomes of the subgroup analysis according to the Δ Lactate 24 h [%]
| Variables | ∆ Lactate 24 h ≤ 0% | ∆ Lactate 24 h > 0% | p value |
|---|---|---|---|
| n = 225 (26%) | n = 634 (74%) | ||
| ICU mortality | 131 (61%) | 303 (50%) | 0.004 |
| 30-day mortality | 128 (62%) | 331 (55%) | 0.077 |
| 3-month mortality | 139 (79%) | 353 (68%) | 0.007 |
| ICU length of stay (hours) | 285 (439) | 348 (400) | 0.029 |
| Duration of mechanical ventilation (hours) | 210 (390) | 277 (340) | 0.106 |
ICU intensive care unit
Fig. 4.
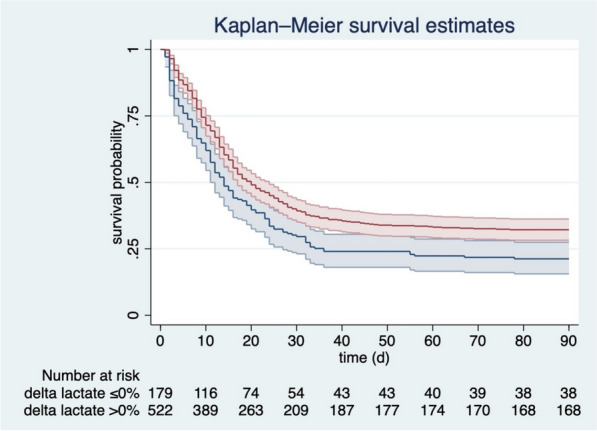
Kaplan–Meier for patients with a ∆ lactate 24 h > 0% (red line) compared to patients with a ∆ lactate 24 h ≤ 0% (blue line) (3-month mortality, ± standard deviation)
Although not statistically significant, ∆ lactate was associated with ICU mortality after multivariable adjustment for age, SOFA score, and gender (aOR 0.997 95% CI 0.993–1.000; p = 0.05). A decreasing lactate (∆ lactate > 0%) was inversely associated with ICU mortality (OR 0.63 95% CI 0.46–0.87; p = 0.004) and remained so after multivariable adjustment for SOFA score, age, CFS and gender (aOR 0.60 95% CI 0.42–0.85; p = 0.004).
Discussion
This sub-group analysis in very old ICU patients with COVID-19 examined the value of baseline lactate and lactate kinetics as predictors of outcome. Both patients with elevated baseline lactate and patients with rising lactate suffered from significantly higher ICU-mortality. These parameters were independently associated with ICU-mortality. To our knowledge, this study represents the first prospective observational study in critically ill old patients with COVID-19 on the value of lactate and lactate kinetics for outcome prediction.
These findings are of pivotal importance as lactate and its kinetics are already amongst the most important laboratory parameters for diagnosing septic shock and guiding treatment. The current sepsis guidelines advocate measurement of lactate at ICU admission and during ICU stay as one of the best variables to assess the response to treatment [14]. Indeed, a meta-analysis by Pan et al. included seven randomized controlled trials with 1301 patients to compare the early lactate clearance-directed therapy to central venous oxygen saturation (ScvO2)-guided therapy as a potentially more effective resuscitation target. They concluded that the use of an early lactate clearance-directed therapy resulted in a decreased in-hospital mortality, shorter ICU stay, shorter mechanical ventilation time, and lower APACHE II scores [15].
COVID-19 might be considered as a very unique type of sepsis. Thus, the “First Update on the Surviving Sepsis Campaign Guidelines on the Management of Adults with Coronavirus Disease 2019 (COVID-19) in the ICU” were developed [9]. In this statement, the authors suggest using dynamic parameters, such as serum lactate measurement, over static parameters to assess fluid responsiveness in adults with COVID-19 and shock, though with a weak level of evidence [9].
Severe COVID-19 is usually characterized by a fast-developing pneumonitis and respiratory failure. Currently, there are only very limited data about serum lactate values in this special type of pneumonitis and in pneumonia in general: Gwak et al. collected consecutive data from 397 patients who were hospitalized with community acquired pneumonia (CAP); 18% of these patients were admitted to the ICU. They found an independent association between the initial serum lactate concentration and in-hospital mortality (aOR1.24; 95% CI 1.01–1.53) [16]. A subgroup analysis of the INFAUCI-Study by Pereira et al. investigated prognostic markers in patients suffering from severe community (in most cases bacterial) acquired pneumonia. In their analysis, the mean serum lactate on admission was higher than in our study (3.0 ± 3.1 mmol/L) and independently associated with intra-hospital mortality (OR 1.13; 95% CI 1.00–1.32, in a sub-group analysis of patients with septic shock: OR 1.11; 95% CI 1.00–1.37, respectively). Interestingly, they found no association with mortality at 6 months [17]. In another retrospective analysis involving 553 patients, Jo et al. showed the non-inferiority of a baseline serum lactate combined with an early warning score compared to established pneumonia scores such as CURB-65 in predicting outcomes of patients with CAP [18].
Regarding serum lactate levels in COVID-19, the present study is in line with Goodall et al., who found a relationship between higher lactate levels (aHR 2.67) and an increased mortality in 981 patients [19]. Kayina et al. examined 235 patients and demonstrated that non-survivors had a higher baseline serum lactate (p < 0.01, n = 122) [20]. In a very small cohort of 45 ICU-patients suffering from COVID-19, Vassiliou et al. found that maximum lactate on admission was independently related to 28-day ICU-mortality. Lactate’s area under the curve for detecting 28-day ICU mortality was 0.77 (p = 0.008). Mixed model analysis showed that mean daily lactate levels were higher in non-survivors (p < 0.0001). Interestingly, when lactate levels were compared to the SOFA scores they showed a similar time pattern [21]. A retrospective cohort study by Gregoriano et al. included 99 patients with severe COVID-19. In this cohort, lactate on admission was amongst the highest prognostic factors for severe COVID-19 progression (lactate on ambient air AUC 0.67; lactate with O2 supply AUC 0.70) [22].
However, the present study also reveals another very relevant insight into old ICU patients with COVID-19: almost 70% of patients had no elevated lactate at all on admission. This contradicts previous studies, such as by Li et al. [23]. These found, in 204 older patients (≥ 60 years) diagnosed with COVID-19, an elevated lactate (median 2.3 mmol/L) in 84% of the patients [23].
Limitations
Our study has some methodological limitations. We lack a control group of younger COVID-19 patients for comparison or a comparable age cohort of patients who were not or could not be admitted to the ICU. In addition, the COVIP database does not capture information on time from symptoms onset to ICU admission, from pre-ICU care and triage or from and level of care, while in the ICU (e.g., nurse-to-patient ratio). These treatment limitations may affect the care of older ICU patients [24]. Participating countries varied widely in their care structure. This results in a large heterogeneity of treatments. The study also cannot answer whether lactate kinetics-guided therapy prospectively gives a mortality benefit in critically ill septic patients with COVID-19. When lactate values were documented, only the first 48 h were recorded, with the highest value per 24 h in each case. However, these parameters have been used as a benchmark in most studies to date, so the determination seems more than adequate to answer the hypothesis. The ∆ Lactate subgroup analysis lacks patients who died in the first 24 h. However, this accounted only for 32 patients of the study cohort. Pre-existing liver disease might influence lactate and its kinetics. Nevertheless, in the multivariate analysis, results have been adjusted to SOFA score which includes liver function. Possibly, patients with pulmonary artery embolism are more frequently in shock leading to elevated lactate. Though, this association is speculative as the study did not investigate the occurrence of pulmonary artery embolism as there were no reports of clustered pulmonary artery emboli when establishing the COVIP study design in February 2020.
Conclusion
In critically ill old intensive care patients suffering from COVID-19, most critically ill old COVID-19 patients had normal serum lactate on admission. However, in those who had an elevated lactate, lactate and lactate kinetics are valuable tools for outcome prediction.
Supplementary Information
Additional file 1. List of Collaborators: COVIP-Study.
Acknowledgements
The COVIP study group consists of the authors and the following persons: Philipp Eller, Michael Joannidis, Dieter Mesotten, Pascal Reper, Sandra Oeyen, Walter Swinnen, Nicolas Serck, Elisabeth Dewaele, Edwin Chapeta, Helene Brix, Jens Brushoej, Pritpal Kumar, Helene Korvenius Nedergaard, Tim Koch Johnsen, Camilla Bundesen, Maria Aagaard Hansen, Stine Uhrenholt, Helle Bundgaard, Jesper Fjølner, Richard Innes, James Gooch, Lenka Cagova, Elizabeth Potter, Michael Reay, Miriam Davey, Mohammed Abdelshafy Abusayed, Sally Humphreys, Amy Collins, Avinash Aujayeb, Susannah Leaver, Waqas Khaliq, Ayman Abdelmawgoad Habib, Mohammed A Azab, Kyrillos Wassim, Yumna A. Elgazzar, Rehab Salah, Hazem Maarouf Abosheaishaa, Aliae AR Mohamed Hussein, Ahmed Y. Azzam, Samar Tharwat, Yasmin Khairy Nasreldin Mohamed Ali, Omar Elmandouh, Islam Galal, Ahmed Abu-Elfatth, Karam Motawea, Mohammad Elbahnasawy, Mostafa Shehata, Mohamed Elbahnasawy, Mostafa Tayeb, Nermin Osman, Wafaa Abdel-Elsalam, Aliae Mohamed Hussein, Amer Aldhalia, Arnaud Galbois, Bertrand Guidet, Cyril Charron, Caroline Hauw Berlemont, Guillaume Besch, Jean-Philippe Rigaud, Julien Maizel, Michel Djibré, Philippe Burtin, Pierre Garcon, Saad Nseir, Xavier Valette, Nica Alexandru, Nathalie Marin, Marie Vaissiere, Gaëtan Plantefeve, Hervé Mentec, Thierry Vanderlinden, Igor Jurcisin, Buno Megarbane, Benjamin Glenn Chousterman, François Dépret, Marc Garnier, Sebastien Besset, Johanna Oziel, Alexis Ferre, Stéphane Dauger, Guillaume Dumas, Bruno Goncalves, Lucie Vettoretti, Didier Thevenin, Stefan Schaller, Muhammed Kurt, Andreas Faltlhauser, Christian Meyer, Milena Milovanovic, Matthias Lutz, Gonxhe Shala, Hendrik Haake, Winfried Randerath, Anselm Kunstein, Patrick Meybohm, Stephan Steiner, Eberhard Barth, Tudor Poerner, Philipp Simon, Marco Lorenz, Zouhir Dindane, Karl Friedrich Kuhn, Martin Welte, Ingo Voigt, Hans-Joachim Kabitz, Jakob Wollborn, Ulrich Goebel, Sandra Emily Stoll, Detlef Kindgen-Milles, Simon Dubler, Christian Jung, Kristina Fuest, Michael Schuster, Stephan Steiner, Antonios Papadogoulas, Francesk Mulita, Nikoletta Rovina, Zoi Aidoni, Evangelia Chrisanthopoulou, Eumorfia Kondili, Ioannis Andrianopoulos, Mohan Gurjar, Ata Mahmoodpoor, Rand Hussein, Maytham Aqeel Al-Juaifari, Abdullah Khudhur Ahmed Karantenachy, Sigal Sviri, Ahmed Elsaka, Brian Marsh, Vittoria Comellini, Farah Al-Ali, Sari Almani, Almu´Atasim Khamees, Khayry Al-Shami, Ibrahim Salah El Din, Taha Abubaker, Hazem Ahmed, Ahmed Rabha, Abdulmueti Alhadi, Marwa Emhamed, Saedah Abdeewi, Abdurraouf Abusalama, Abdulmueti Alhadi, Mohammed Huwaysh, Esraa Abdalqader Alghati, Abdelilah Ghannam, Silvio A Namendys-Sylva, Martijn Groenendijk, Mirjam Evers, Lenneke Van Lelyveld-Haas, Iwan Meynaar, Alexander Daniel Cornet, Marieke Zegers, Willem Dieperink, Dylan De Lange, Tom Dormans, Michael Hahn, Britt Sjøbøe, Hans Frank Strietzel, Theresa Olasveengen, Luis Romundstad, Finn H. Andersen, John George Grace Massoud, Aamir Ghafoor Khan, Shahd Al-Qasrawi, Sarah Amro, Anna Kluzik, Paweł Zatorski, Tomasz Drygalski, Wojciech Szczeklik, Jakub Klimkiewicz, Joanna Solek-Pastuszka, Dariusz Onichimowski, Miroslaw Czuczwar, Ryszard Gawda, Jan Stefaniak, Karina Stefanska-Wronka, Ewa Zabul, Ana Isabel Pinho Oliveira, Rui Assis, Maria De Lurdes Campos Santos, Henrique Santos, Filipe Sousa Cardoso, André Gordinho, Ioana Marina Grintescu, Dana Tomescu, Mohamed Raafat Badawy, M José Arche Banzo, Begoña Zalba-Etayo, Patricia Jimeno Cubero, Jesús Priego, Gemma Gomà, Teresa Maria Tomasa-Irriguible, Susana Sancho, Aida Fernández Ferreira, Eric Mayor Vázquez, Ángela Prado Mira, Mercedes Ibarz, David Iglesias, Susana Arias-Rivera, Fernando Frutos-Vivar, Sonia Lopez-Cuenca, Cesar Aldecoa, David Perez-Torres, Isabel Canas-Perez, Luis Tamayo-Lomas, Cristina Diaz-Rodriguez, Pablo Ruiz De Gopegui, Mahmoud Saleh, Momin Majed Yousuf Hilles, Enas M. Y Abualqumboz, Nawfel Ben-Hamouda, Andrea Roberti, Yvan Fleury, Nour Abidi, Joerg C. Schefold, Ivan Chau, Alexander Dullenkopf, Mohammad Karam Chaaban, Mohammed Mouaz Shebani, Ahmad Hmaideh, Aymen Shaher, Ayca Sultan Sahin, Kemal Tolga Saracoglu, Mohammed Al-Sadawi, Richard Pugh, Sara Smuts and Rafat Ameen Mohammed Al-Saban.
Authors’ contributions
BW, RRB and CJ analyzed the data and wrote the first draft of the manuscript. HF and BG and DL and IS contributed to statistical analysis and improved the paper. SB and PHB and JM and MK and AB and AM and FA and AA and BG and MC and SC and LF and JF and ML and BM and RM and SO and CÖ and BBP and MAB and WS and AV and CW and TZ and JCS and JW gave guidance and improved the paper. All authors read and approved the final manuscript.
Funding
Open Access funding enabled and organized by Projekt DEAL. This study was endorsed by the ESICM. Free support for running the electronic database and was granted from the dep. of Epidemiology, University of Aarhus, Denmark. The support of the study in France by a Grant from Fondation Assistance Publique-Hôpitaux de Paris pour la recherche is greatly appreciated. In Norway, the study was supported by a grant from the Health Region West. In addition, the study was supported by a Grant from the European Open Science Cloud (EOSC). EOSCsecretariat.eu has received funding from the European Union’s Horizon Programme call H2020-INFRAEOSC-05-2018-2019, Grant Agreement Number 831644. This work was supported by the Forschungskommission of the Medical Faculty of the Heinrich-Heine-University Düsseldorf, and No. 2020-21 to RRB for a Clinician Scientist Track.
Availability of data and materials
Individual participant data that underlie the results reported in this article are available to investigators whose proposed use of the data has been approved by the COVIP steering committee. The anonymized data can be requested from the authors if required.
Declarations
Ethics approval and consent to participate
The primary competent ethics committee was the Ethics Committee of the University of Duesseldorf, Germany. Institutional research ethic board approval was obtained from each study site.
Consent for publication
The manuscript does not contain any individual person’s data in any form.
Competing interests
The authors declare that they have no competing interests. JCS reports grants (full departmental disclosure) from Orion Pharma, Abbott Nutrition International, B. Braun Medical AG, CSEM AG, Edwards Lifesciences Services GmbH, Kenta Biotech Ltd, Maquet Critical Care AB, Omnicare Clinical Research AG, Nestle, Pierre Fabre Pharma AG, Pfizer, Bard Medica S.A., Abbott AG, Anandic Medical Systems, Pan Gas AG Healthcare, Bracco, Hamilton Medical AG, Fresenius Kabi, Getinge Group Maquet AG, Dräger AG, Teleflex Medical GmbH, Glaxo Smith Kline, Merck Sharp and Dohme AG, Eli Lilly and Company, Baxter, Astellas, Astra Zeneca, CSL Behring, Novartis, Covidien, Philips Medical, Phagenesis Ltd, Prolong Pharmaceuticals and Nycomed outside the submitted work. The money went into departmental funds. No personal financial gain applied.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Raphael Romano Bruno and Bernhard Wernly have contributed equally
Contributor Information
Raphael Romano Bruno, Email: Raphael.Bruno@med.uni-duesseldorf.de.
Bernhard Wernly, Email: bernhard@wernly.ate.
Hans Flaatten, Email: Hans.Flaatten@uib.no.
Jesper Fjølner, Email: Jespfjoe@rm.dk.
Antonio Artigas, Email: aartigas@tauli.cat.
Bernardo Bollen Pinto, Email: bollenpinto@gmail.com.
Joerg C. Schefold, Email: joerg.schefold@insel.ch
Stephan Binnebössel, Email: stephan.binneboessel@med.uni-duesseldorf.de.
Philipp Heinrich Baldia, Email: PhilippHeinrich.Baldia@med.uni-duesseldorf.de.
Malte Kelm, Email: Malte.Kelm@med.uni-duesseldorf.de.
Michael Beil, Email: beil@doctors.org.uk.
Sivri Sigal, Email: sigals@hadassah.org.il.
Peter Vernon van Heerden, Email: vernon@hadassah.org.il.
Wojciech Szczeklik, Email: wojciech.szczeklik@uj.edu.pl.
Muhammed Elhadi, Email: muhammed.elhadi.uot@gmail.com.
Michael Joannidis, Email: michael.joannidis@tirol-kliniken.at.
Sandra Oeyen, Email: sandra.oeyen@ugent.be.
Tilemachos Zafeiridis, Email: tilemachos@hotmail.com.
Jakob Wollborn, Email: jwollborn@bwh.harvard.edu.
Maria José Arche Banzo, Email: mariajosearchebanzo@gmail.com.
Kristina Fuest, Email: kristina.fuest@tum.de.
Brian Marsh, Email: bmarsh@mater.ie.
Finn H. Andersen, Email: finn.h.andersen@helse-mr.no
Rui Moreno, Email: r.moreno@mail.telepac.pt.
Susannah Leaver, Email: Ccrg@stgeorges.nhs.uk.
Ariane Boumendil, Email: ariane.boumendil@gmail.com.
Dylan W. De Lange, Email: d.w.delange@umcutrecht.nl
Bertrand Guidet, Email: bertrand.guiet@aphp.fr.
Christian Jung, Email: christian.jung@med.uni-duesseldorf.de.
the COVIP Study Group:
Philipp Eller, Michael Joannidis, Dieter Mesotten, Pascal Reper, Sandra Oeyen, Walter Swinnen, Nicolas Serck, Elisabeth Dewaele, Edwin Chapeta, Helene Brix, Jens Brushoej, Pritpal Kumar, Helene Korvenius Nedergaard, Tim Koch Johnsen, Camilla Bundesen, Maria Aagaard Hansen, Stine Uhrenholt, Helle Bundgaard, Jesper Fjølner, Richard Innes, James Gooch, Lenka Cagova, Elizabeth Potter, Michael Reay, Miriam Davey, Mohammed Abdelshafy Abusayed, Sally Humphreys, Amy Collins, Avinash Aujayeb, Susannah Leaver, Waqas Khaliq, Ayman Abdelmawgoad Habib, Mohammed A. Azab, Kyrillos Wassim, Yumna A. Elgazzar, Rehab Salah, Hazem Maarouf Abosheaishaa, Aliae A. R. Hussein Mohamed, Ahmed Y. Azzam, Samar Tharwat, Yasmin Khairy Nasreldin Mohamed Ali, Omar Elmandouh, Islam Galal, Ahmed Abu-Elfatth, Karam Motawea, Mohammad Elbahnasawy, Mostafa Shehata, Mohamed Elbahnasawy, Mostafa Tayeb, Nermin Osman, Wafaa Abdel-Elsalam, Aliae Mohamed Hussein, Amer Aldhalia, Arnaud Galbois, Bertrand Guidet, Cyril Charron, Caroline Hauw Berlemont, Guillaume Besch, Jean-Philippe Rigaud, Julien Maizel, Michel Djibré, Philippe Burtin, Pierre Garcon, Saad Nseir, Xavier Valette, Nica Alexandru, Nathalie Marin, Marie Vaissiere, Gaëtan Plantefeve, Hervé Mentec, Thierry Vanderlinden, Igor Jurcisin, Buno Megarbane, Benjamin Glenn Chousterman, François Dépret, Marc Garnier, Sebastien Besset, Johanna Oziel, Alexis Ferre, Stéphane Dauger, Guillaume Dumas, Bruno Goncalves, Lucie Vettoretti, Didier Thevenin, Stefan Schaller, Muhammed Kurt, Andreas Faltlhauser, Christian Meyer, Milena Milovanovic, Matthias Lutz, Gonxhe Shala, Hendrik Haake, Winfried Randerath, Anselm Kunstein, Patrick Meybohm, Stephan Steiner, Eberhard Barth, Tudor Poerner, Philipp Simon, Marco Lorenz, Zouhir Dindane, Karl Friedrich Kuhn, Martin Welte, Ingo Voigt, Hans-Joachim Kabitz, Jakob Wollborn, Ulrich Goebel, Sandra Emily Stoll, Detlef Kindgen-Milles, Simon Dubler, Christian Jung, Kristina Fuest, Michael Schuster, Stephan Steiner, Antonios Papadogoulas, Francesk Mulita, Nikoletta Rovina, Zoi Aidoni, Evangelia Chrisanthopoulou, Eumorfia Kondili, Ioannis Andrianopoulos, Mohan Gurjar, Ata Mahmoodpoor, Rand Hussein, Maytham Aqeel Al-Juaifari, Abdullah Khudhur Ahmed Karantenachy, Sigal Sviri, Ahmed Elsaka, Brian Marsh, Vittoria Comellini, Farah Al-Ali, Sari Almani, Almu’ Atasim Khamees, Khayry Al-Shami, Ibrahim Salah El Din, Taha Abubaker, Hazem Ahmed, Ahmed Rabha, Abdulmueti Alhadi, Marwa Emhamed, Saedah Abdeewi, Abdurraouf Abusalama, Abdulmueti Alhadi, Mohammed Huwaysh, Esraa Abdalqader Alghati, Abdelilah Ghannam, Silvio A. Namendys-Sylva, Martijn Groenendijk, Mirjam Evers, Lenneke Van Lelyveld-Haas, Iwan Meynaar, Alexander Daniel Cornet, Marieke Zegers, Willem Dieperink, Dylan De Lange, Tom Dormans, Michael Hahn, Britt Sjøbøe, Hans Frank Strietzel, Theresa Olasveengen, Luis Romundstad, Finn H. Andersen, John George Grace Massoud, Aamir Ghafoor Khan, Shahd Al-Qasrawi, Sarah Amro, Anna Kluzik, Paweł Zatorski, Tomasz Drygalski, Wojciech Szczeklik, Jakub Klimkiewicz, Joanna Solek-Pastuszka, Dariusz Onichimowski, Miroslaw Czuczwar, Ryszard Gawda, Jan Stefaniak, Karina Stefanska-Wronka, Ewa Zabul, Ana Isabel Pinho Oliveira, Rui Assis, Maria De Lurdes Campos Santos, Henrique Santos, Filipe Sousa Cardoso, André Gordinho, Ioana Marina Grintescu, Dana Tomescu, Mohamed Raafat Badawy, M. José Arche Banzo, Begoña Zalba-Etayo, Patricia Jimeno Cubero, Jesús Priego, Gemma Gomà, Teresa Maria Tomasa-Irriguible, Susana Sancho, Aida Fernández Ferreira, Eric Mayor Vázquez, Ángela Prado Mira, Mercedes Ibarz, David Iglesias, Susana Arias-Rivera, Fernando Frutos-Vivar, Sonia Lopez-Cuenca, Cesar Aldecoa, David Perez-Torres, Isabel Canas-Perez, Luis Tamayo-Lomas, Cristina Diaz-Rodriguez, Pablo Ruiz De Gopegui, Mahmoud Saleh, Momin Majed Yousuf Hilles, Enas M. Y. Abualqumboz, Nawfel Ben-Hamouda, Andrea Roberti, Yvan Fleury, Nour Abidi, Joerg C. Schefold, Ivan Chau, Alexander Dullenkopf, Mohammad Karam Chaaban, Mohammed Mouaz Shebani, Ahmad Hmaideh, Aymen Shaher, Ayca Sultan Sahin, Kemal Tolga Saracoglu, Mohammed Al-Sadawi, Richard Pugh, Sara Smuts, and Rafat Ameen Mohammed Al-Saban
References
- 1.Armstrong RA, Kane AD, Cook TM. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75(10):1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 2.Bellelli G, Rebora P, Valsecchi MG, Bonfanti P, Citerio G, Members C-MT Frailty index predicts poor outcome in COVID-19 patients. Intensive Care Med. 2020;46(8):1634–1636. doi: 10.1007/s00134-020-06087-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jung C, Flaatten H, Fjolner J, et al. The impact of frailty on survival in elderly intensive care patients with COVID-19: the COVIP study. Crit Care. 2021;25(1):149. doi: 10.1186/s13054-021-03551-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Masyuk M, Wernly B, Lichtenauer M, et al. Prognostic relevance of serum lactate kinetics in critically ill patients. Intensive Care Med. 2019;45(1):55–61. doi: 10.1007/s00134-018-5475-3. [DOI] [PubMed] [Google Scholar]
- 5.Bruno RR, Wernly B, Binneboessel S, et al. Failure of lactate clearance predicts the outcome of critically ill septic patients. Diagnostics. 2020 doi: 10.3390/diagnostics10121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hernandez G, Bellomo R, Bakker J. The ten pitfalls of lactate clearance in sepsis. Intensive Care Med. 2019;45(1):82–85. doi: 10.1007/s00134-018-5213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wernly B, Bakker J, Jung C. Venous blood lactate concentrations in patients with shock: Interesting but not really helpful. J Crit Care. 2020;58:125–126. doi: 10.1016/j.jcrc.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3) JAMA. 2016;315(8):801–810. doi: 10.1001/jama.2016.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alhazzani W, Evans L, Alshamsi F, et al. Surviving sepsis campaign guidelines on the management of adults with coronavirus disease 2019 (COVID-19) in the ICU: 55. Crit Care Med. 2021;49(3):e219–34. doi: 10.1097/CCM.0000000000004899. [DOI] [PubMed] [Google Scholar]
- 10.Phua J, Weng L, Ling L, et al. Intensive care management of coronavirus disease 2019 (COVID-19): challenges and recommendations. Lancet Respir Med. 2020;8(5):506–517. doi: 10.1016/S2213-2600(20)30161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Smorenberg A, Peters EJG, van Daele PLA, Nossent EJ, Muller M. How does SARS-CoV-2 targets the elderly patients? A review on potential mechanisms increasing disease severity. Eur J Intern Med. 2021;83:1–5. doi: 10.1016/j.ejim.2020.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flaatten H, De Lange DW, Morandi A, et al. The impact of frailty on ICU and 30-day mortality and the level of care in very elderly patients (≥ 80 years) Intensive Care Med. 2017;43(12):1820–1828. doi: 10.1007/s00134-017-4940-8. [DOI] [PubMed] [Google Scholar]
- 13.Guidet B, de Lange DW, Boumendil A, et al. The contribution of frailty, cognition, activity of daily life and comorbidities on outcome in acutely admitted patients over 80 years in European ICUs: the VIP2 study. Intensive Care Med. 2020;46(1):57–69. doi: 10.1007/s00134-019-05853-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellinger RP, Levy MM, Carlet JM, et al. Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med. 2008;36(1):296–327. doi: 10.1097/01.CCM.0000298158.12101.41. [DOI] [PubMed] [Google Scholar]
- 15.Pan J, Peng M, Liao C, Hu X, Wang A, Li X. Relative efficacy and safety of early lactate clearance-guided therapy resuscitation in patients with sepsis: a meta-analysis. Medicine. 2019;98(8):e14453. doi: 10.1097/MD.0000000000014453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gwak MH, Jo S, Jeong T, et al. Initial serum lactate level is associated with inpatient mortality in patients with community-acquired pneumonia. Am J Emerg Med. 2015;33(5):685–690. doi: 10.1016/j.ajem.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 17.Pereira JM, Goncalves-Pereira J, Ribeiro O, Baptista JP, Froes F, Paiva JA. Impact of antibiotic therapy in severe community-acquired pneumonia: data from the Infauci study. J Crit Care. 2018;43:183–189. doi: 10.1016/j.jcrc.2017.08.048. [DOI] [PubMed] [Google Scholar]
- 18.Jo S, Jeong T, Lee JB, Jin Y, Yoon J, Park B. Validation of modified early warning score using serum lactate level in community-acquired pneumonia patients. The national early warning score-lactate score. Am J Emerg Med. 2016;34(3):536–541. doi: 10.1016/j.ajem.2015.12.067. [DOI] [PubMed] [Google Scholar]
- 19.Goodall JW, Reed TAN, Ardissino M, et al. Risk factors for severe disease in patients admitted with COVID-19 to a hospital in London, England: a retrospective cohort study. Epidemiol Infect. 2020;148:e251. doi: 10.1017/S0950268820002472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayina CA, Haritha D, Soni L, et al. Epidemiological & clinical characteristics & early outcome of COVID-19 patients in a tertiary care teaching hospital in India: a preliminary analysis. Indian J Med Res. 2020;152(1 & 2):100–104. doi: 10.4103/ijmr.IJMR_2890_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassiliou AG, Jahaj E, Ilias I, et al. Lactate kinetics reflect organ dysfunction and are associated with adverse outcomes in intensive care unit patients with COVID-19 pneumonia: preliminary results from a GREEK single-centre study. Metabolites. 2020 doi: 10.3390/metabo10100386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gregoriano C, Koch D, Haubitz S, et al. Characteristics, predictors and outcomes among 99 patients hospitalised with COVID-19 in a tertiary care centre in Switzerland: an observational analysis. Swiss Med Wkly. 2020;150:w20316. doi: 10.4414/smw.2020.20316. [DOI] [PubMed] [Google Scholar]
- 23.Li P, Chen L, Liu Z, et al. Clinical features and short-term outcomes of elderly patients with COVID-19. Int J Infect Dis. 2020;97:245–250. doi: 10.1016/j.ijid.2020.05.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Flaatten H, deLange D, Jung C, Beil M, Guidet B. The impact of end-of-life care on ICU outcome. Intensive Care Med. 2021 doi: 10.1007/s00134-021-06365-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. List of Collaborators: COVIP-Study.
Data Availability Statement
Individual participant data that underlie the results reported in this article are available to investigators whose proposed use of the data has been approved by the COVIP steering committee. The anonymized data can be requested from the authors if required.