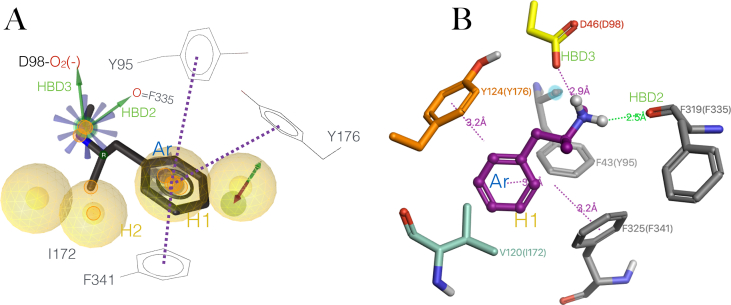
Figure 8.
Binding modes of Methamphetamine on the hSERT and on the dDAT (A) Proposed active conformation of Methamphetamine in black, stick representation aligned to the pharmacophore hypothesis based on MDMA congenerics to the hSERT, showing the interacting residues and functional groups. HBDs.- Hbond donors in green arrows, Ar.-aromatic π-π interactions in discontinued purple lines, H.- hydrophobic interactions in yellow spheres. (B) Binding mode of Methamphetamine (in purple) from the X-ray complex with D. melanogaster dopamine transporter (PDB: 4XP6), the residues are numbered from the original structure and in parenthesis the corresponding residues of hSERT upon sequence alignment. Interactions associated to their distances. In purple edge-to-face π-π stackings and a salt bridge with the acidic residue (in yellow), an Hbond in green, some features of the pharmacophore are identified e.g HBD3, HBDS, yet highlighted in cyan the absence of HBD1 with the side chain of residue corresponding to Tyr95.