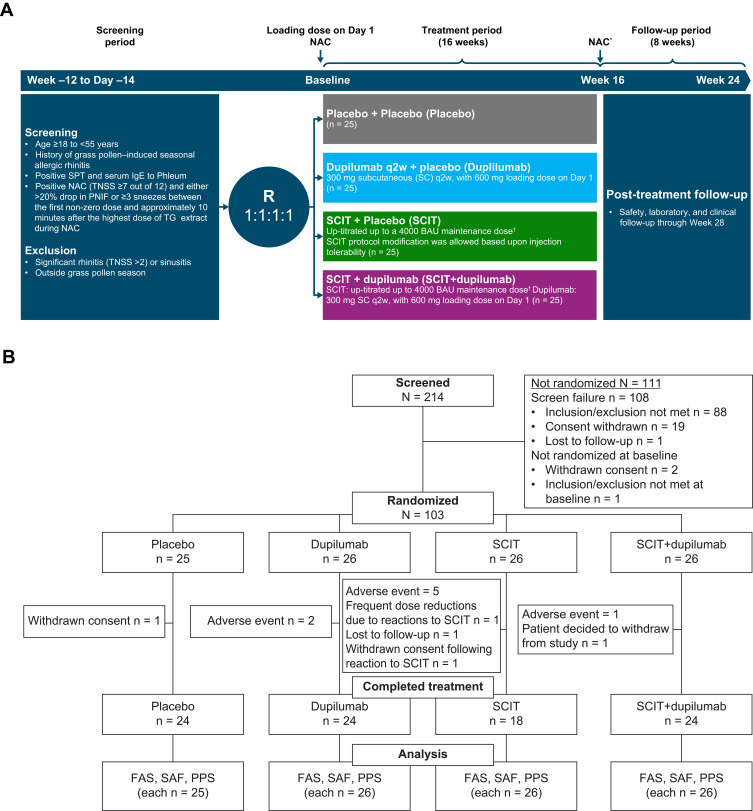
Figure 1.
Study information. (A) CONSORT diagram of patient disposition and (B) study design. *NAC occurred at Week 17, after 16 weeks of treatment. †SCIT dosing regimen is detailed in Supplementary Table 1 in this article’s Online Repository at https://www.dovepress.com/.
Abbreviations: BAU, bioequivalent allergy unit; FAS, full analysis set; NAC, nasal allergen challenge; PNIF, peak nasal inspiratory flow; PPS, per protocol set; q2w, every 2 weeks; R, randomization; SAF, safety analysis set; SC, subcutaneous; SCIT, subcutaneous immunotherapy; SPT, skin prick test; TG, timothy grass; TNSS, total nasal symptom score.