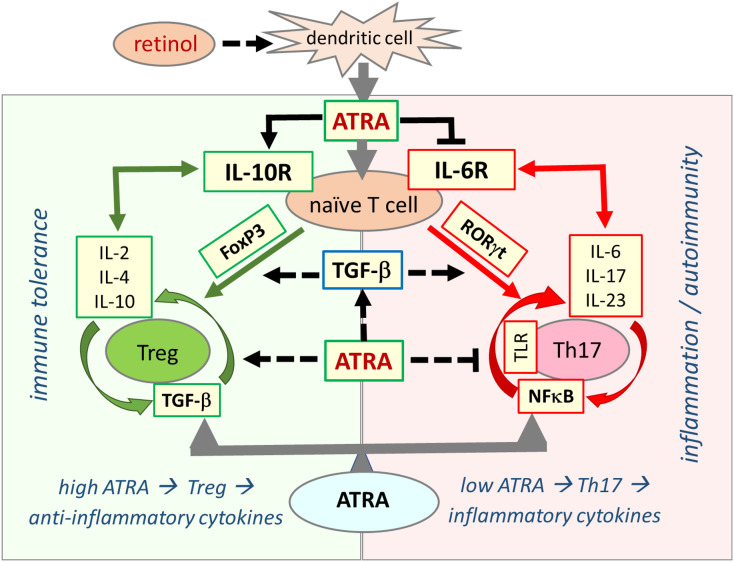
Fig. 2.
Retinoic acid signaling is essential in establishing immune tolerance through promoting differentiation of Tregs and inhibiting development of proinflammatory Th17 cells. The balance between Treg and Th17 cells defines immune tolerance or inflammation. Both Treg and Th17 cells differentiate from naive T cells under different cellular and tissue milieu requiring TGF-β. In the presence of sufficient ATRA levels, the interaction between signaling pathways from RA, TGF-β, and cytokines blocks IL-6 signaling and induce FoxP3 transcription factor that ultimately leads to differentiation of naïve T cells into Tregs, which induce expression of anti-inflammatory cytokines, such as IL-2, IL-4, and IL-10, and suppress the inflammatory response that culminate in establishment of self-tolerance (immune tolerance circle, green circled arrows, left side panel). In the presence of low or no ATRA, retinoid signaling is weak or absent and fails to block IL-6 signaling, leading to the expression and activation of RORγt that reprogram differentiation of naïve T cells into proinflammatory Th17 cells. Effector Th17 cells have high activity of NF-κB signaling that leads to expression and release of various proinflammatory cytokines such as IL-6, IL-17, and IL-23, generating an inflammatory response that may lead to development of autoimmune diseases (inflammation circle, red circled arrows, right side panel). ATRA exerts its effect by inducing expression of TGF-β and FoxP3 (dotted black arrows) and inhibiting activation of NF-κB (blunt-ended dotted black line). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)