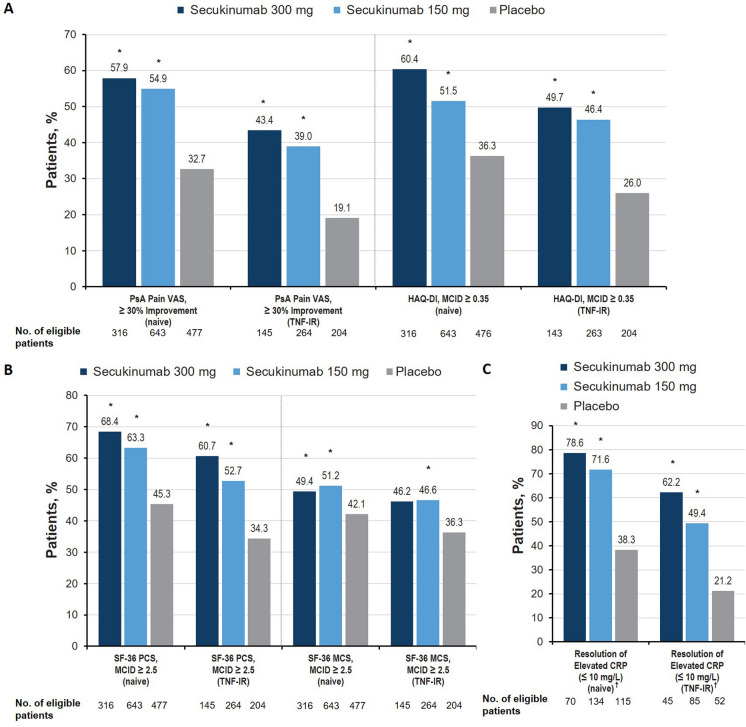
Fig. 3.
Percentage of patients with meaningful improvement in the a pain and physical function, b health-related quality of life, and c systemic inflammation domains at week 16 in the FUTURE 2–5 trials. CRP C-reactive protein, LSM least squares mean, MCID minimal clinically important difference, MCS mental component summary, PCS physical component summary, PsA psoriatic arthritis, SF-36 36-item Short Form Health Survey, TNF-IR tumor necrosis factor inhibitor inadequate responder, VAS visual analog scale. †Among patients with CRP greater than 10 mg/L at baseline. *P < 0.05 vs placebo