Figure 5. RagA or RagC are not required for NT-GSDMD plasma membrane localization, but are required for oligomerization and pore formation.
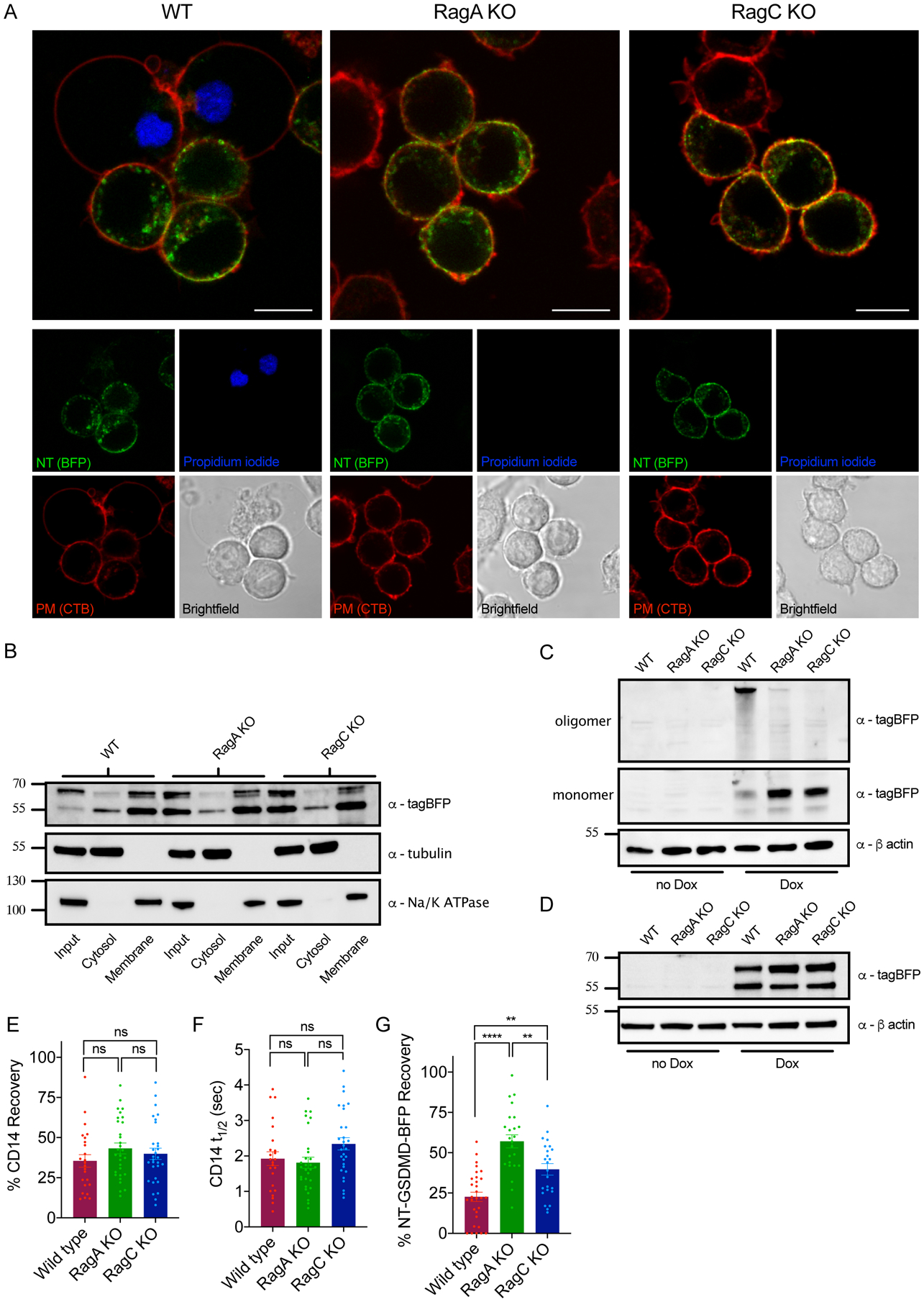
(A) Confocal microscopy of NT-GSDMD-BFP (green) at 8 hours post Dox-induction (0.5 μg/ml) in WT, RagA KO, and RagC KO cells. Plasma membrane was labeled with CTB-AF647 (red) and membrane permeability was assessed by PI (blue). Scale bar indicates 10 μm.
(B) Western blot of NT-GSDMD-BFP from post-nuclear input, cytosolic, and bulk membrane fractions of WT, RagA KO, and RagC KO cells, after Dox-induction (2 μg/ml) for 8 hours. Tubulin represents a cytosol marker and Na+/K+ ATPase represents a membrane marker.
(C, D) Western blot of NT-GSDMD-BFP from combined cell lysate and supernatant under non-reducing conditions (C) or reducing conditions (D) from WT, RagA KO, and RagC KO cells after Dox-induction for 16 hours.
(E) FRAP proportion of fluorescence recovery of CD14 labeled with PE-conjugated anti-CD14 antibody in WT, RagA KO, and RagC KO macrophages.
(F) FRAP half-life of fluorescence recovery of CD14 labled with PE-conjugated anti-CD14 antibody in WT, RagA KO, and RagC KO macrophages.
(G) FRAP proportion of fluorescence recovery of BFP signal from NT-GSDMD-BFP in WT, RagA KO, and RagC KO macrophages after Dox-induction (2 μg/ml) for 8 hours.
Each data point (E, F, G) represents parameters from a fitted FRAP curve from individual cells (n ≥ 24). One-way ANOVA was used for analysis.
