Figure 7. Mutation of position Q3.32 differentially affects peptide binding at NPR-1 and NPR-11 and switches the binding mode of FLP-21 at NPR-11.
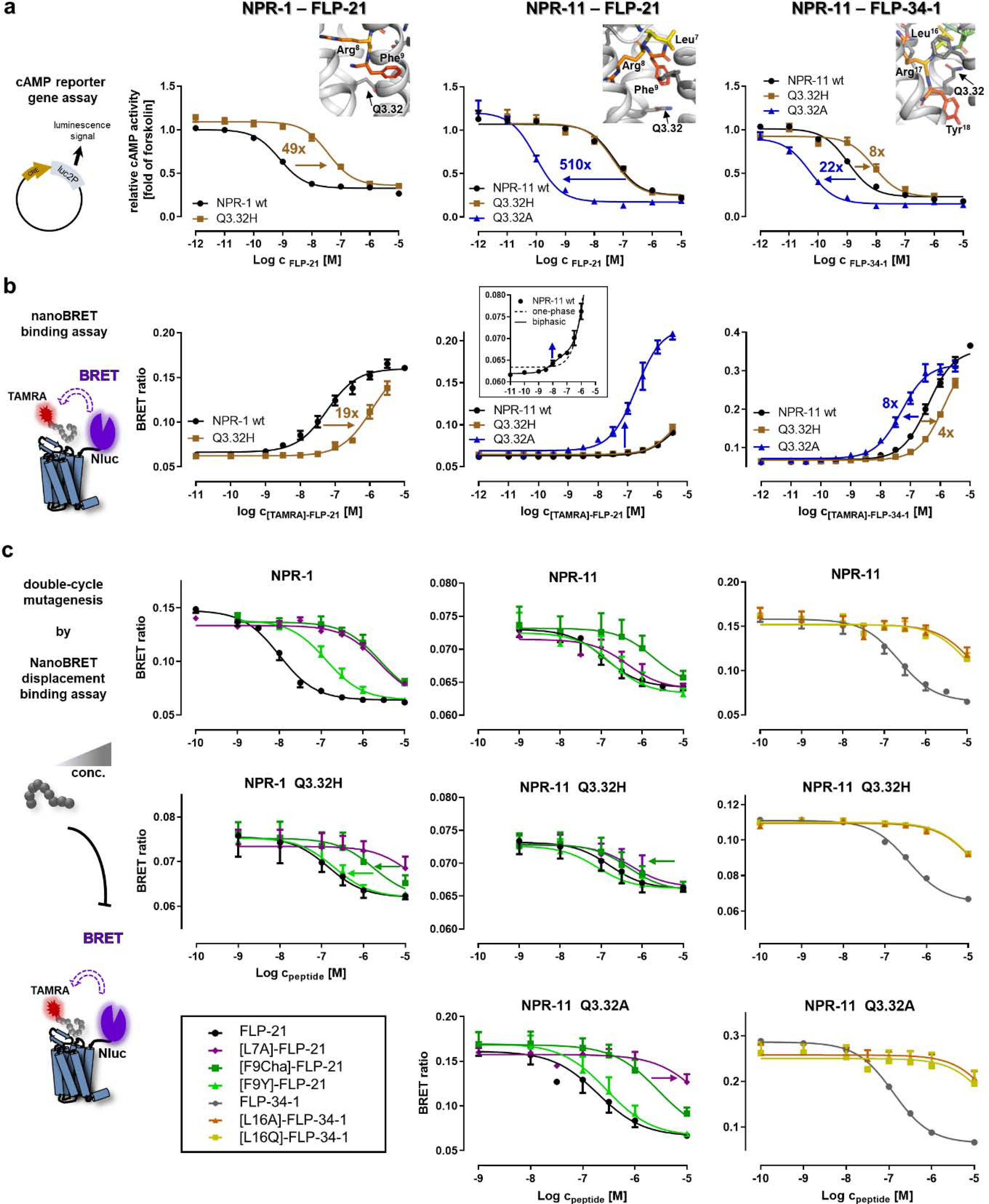
a) Receptor position Q3.32 was tested in a cAMP reporter gene assay (Gi/o) for all three peptide–receptor combinations (from left to right, NPR-1 – FLP-21, NPR-11 – FLP-21 and NPR-11 – FLP-34–1, respectively). The Q3.32A variant of NPR-1 is not correctly folded and was not tested (cf. SI: Figure S1). Insets show the location of Q3.32 in the binding pocket. The Q3.32H variant of NPR-1 displays severely impaired activation. In NPR-11, the Q3.32A exchange induces a dramatic gain of function for FLP-21 and FLP-34–1; while mutation to histidine does not affect activation by FLP-21, but impairs receptor activation by FLP-34–1. b) NanoBRET binding assays to determine peptide affinities at the different receptor variants. Left: NPR-1 mutant Q3.32H displays a weaker affinity to [TAMRA]-FLP-21, in line with the functional data (cf. a). Middle: wild type NPR-11 displays a biphasic behavior for binding of [TAMRA]-FLP-21 with a high-affinity, but low-BRET efficiency component (Kd 15 nM) and a very low-affinity component with a Kd >5 μM. The Q3.32A variant displays a dramatically increased BRET window with a Kd of 190 nM, suggesting a change of binding orientation. Right: The Q3.32H variant of NPR-11 reduces affinity of [TAMRA]-FLP-34–1, while the Q3.32A variant has a moderately increased Kd, without changing the BRET window. c) Displacement binding assays to pinpoint interactions of Q3.32 to the peptides by complementary mutagenesis (cf. Figure 3f). Left: At NPR-1, F9 of FLP-21 interacts with Q3.32, as [F9Y]-FLP-21 (light green triangles) and [F9Cha]-FLP-21 (dark green squares) have a reduced shift of Ki relative to FLP-21 at the Q3.32H variant. In contrast, [L7A]-FLP-21 (purple diamond) has the same Ki shift relative to FLP-21 at both receptor variants. Middle: Binding of FLP-21 variants to NPR-11. F9 of FLP-21 interacts with Q3.32 of NPR-11, as [F9Cha]-FLP-21 (dark green squares) and to a lesser extent [F9Y]-FLP-21 display improved Ki values relative to wild type FLP-21 at the Q3.32H variant of NPR-11. The interactions of L7 of FLP-21 change in the Q3.32A variant of NPR-11 compared to wild type NPR-11 (and Q3.32H variant), as there is a marked rightward shift of the Ki of [L7A]-FLP-21 relative to FLP-21 that is not present at NPR-11 wild type or Q3.32H. Right: L16 of FLP-34 is critical for binding to NPR-11. [L16A/Q]-FLP-34–1 variants (orange triangles and yellow squares, respectively) lose affinity at NPR-11, which is even more pronounced in the Q3.32A mutant. All data represent mean ± SEM of n≥3 independent experiments conducted in technical triplicate.
