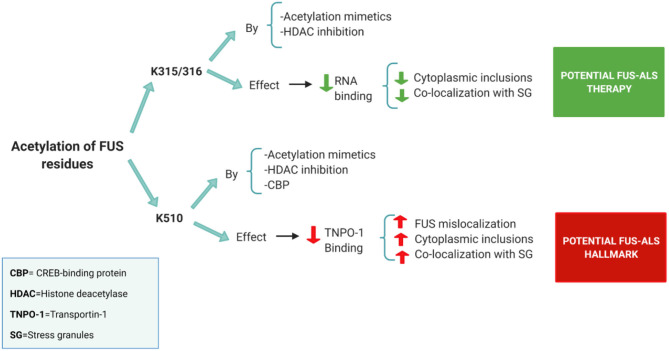
Figure 2.
Summary of the acetylation sites in FUS and its potential effects on FUS-ALS pathology [based on the results obtained in the study of Arenas et al. (2020)]. Acetylation of the FUS RRM (K315/316) by both acetylation mimetics and HDAC pan-inhibition (using a DACi cocktail) decreased FUS ability to bind to RNA. Prevention of RNA binding reduces cytoplasmic inclusions and FUS colocalization with stress granules. Thus, FUS acetylation on RRM sites has a positive effect on FUS-ALS pathology. On the other hand, FUS NLS region (K510 site) proved to be acetylated by acetylation mimetics, CBP or pan-HDAC inhibition. Acetylation of the K510 residue results in the loss of affinity with Transportin-1 leading to increased FUS mislocalization. Thus, FUS acetylation on the NLS site has a negative effect on FUS-ALS pathology.