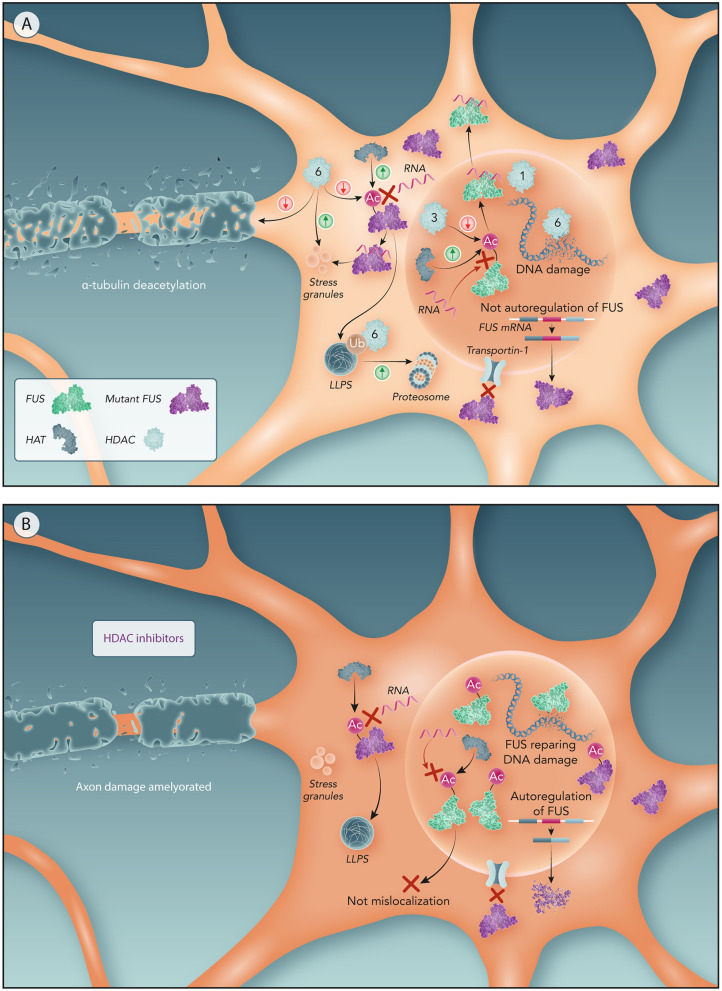
Figure 4.
Potential molecular mechanism of HDACs to drive FUS mislocalization and related ALS features. (A) Motor neurons with NLS-mutated FUS. Mutations in NLS domain impairs FUS binding to Transportin-1 resulting in FUS cytoplasm sequestration. Lower concentrations of nuclear FUS not activates FUS autoregulation resulting in an overproduction of mutant FUS producing further increase on FUS cytoplasmic levels. Nuclear HATs acetylate FUS RRM preventing its binding to RNA and hence FUS egress from the nucleus. Nuclear HDACs (like HDAC3) increases FUS mislocalization by deacetylating RRM. A higher ratio of nuclear HDACs/HATs has been associated with ALS, hence driving FUS mislocalization through HDACs. Moreover, the reduction of HDAC1-FUS interactions caused by FUS mislocalization impairs DNA repair resulting in DNA damage. In the cytoplasm, HATs acetylation of the RRM can promote LLPS of FUS. HDAC6 participates in the proteasomal clearance of FUS aggregates. Abnormal cytoplasmic expression of HDAC6 impairs axonal transport and, also, generates DNA damage. (B) Motor neurons with NLS-mutated FUS treated by HDAC inhibitors. In absence of nuclear HDACs, nuclear FUS is only subject of the HATs effect, thereby acetylation of FUS RRM keeps FUS within the nucleus. Therefore, maintaining FUS in the nucleus reactivates the autoregulatory mechanism of FUS reducing the production of mutant FUS. Prevention of FUS mislocalization also avoids FUS recruitment into stress granules and stress impairments associated to ALS. Moreover, HDAC6 inhibition ameliorates axonal damage from α-tubulin deacetylation and DNA damage caused by HDAC6.