Abstract
Gastric cancer inflicts significant health issues globally despite its declining incidence. The disease is known to be diagnosed at its advanced stages also corresponding with a poor prognosis for patients. The integral therapeutic choices to cure advanced gastric cancer have progressed swiftly in modern days. The preface of molecularly targeted therapeutic techniques would potentiate the personalized approach depending on patient-specific and tumor-specific features, exasperating the advantages of chemotherapy. Here we have reviewed the modern therapeutics such as immune therapy, chemotherapy, m-RNA based therapeutics, alongside evaluating the influence of age, sex and comorbidities-like factors on the occurrence of gastric cancer. Gastric cancer therapy consolidated target agents comprising inhibitors of programmed death-1(PD-1), human epidermal growth factor receptor 2 (HER2), mRNA, and epidermal growth factor receptor (EPGF). A combination of trastuzumab to platinum-mediated chemotherapy evolved has a typical front-line therapy in advanced gastric cancer. An attempt has been made to epitomize the contemporary-modern research on targeted therapy for advanced gastric cancer.
Keywords: Gastric cancer, Therapeutics, MicroRNAs, Chemotherapy, Helicobacter pylori
1. Introduction
Gastric cancer (GC) is a death-dealing disorder leading to have an intimidating impact on global health. Despite a substantial decrease in the rate of incidence over the last few years, gastric cancer still remains the fourth most typical form of cancer, with a recent report of as many as 1,033,701 new cases (in 2018), 782,685 deaths and thus remains as the second leading cause of cancer-related deaths worldwide (Lipitz-Snyderman et al., 2020). The decline in the occurrences of GC are mainly due to improved nutrition, food preservation, pre-symptomatic diagnosis, treatment, and administration of therapeutics. However, the disorder is still known to show a poor prognosis (Lv et al., 2019). The ubiquity of GC has a significant regional difference, as more than half of all newly reported cases of gastric cancer are diagnosed in Japan, East Asia, including Korea and China (Yu et al., 2016) (see Fig. 1, Fig. 2, Fig. 3).
Fig. 1.
Mechanistic action of immune-check point inhibitors (Ishikawa et al., 2018, Park et al., 2019).
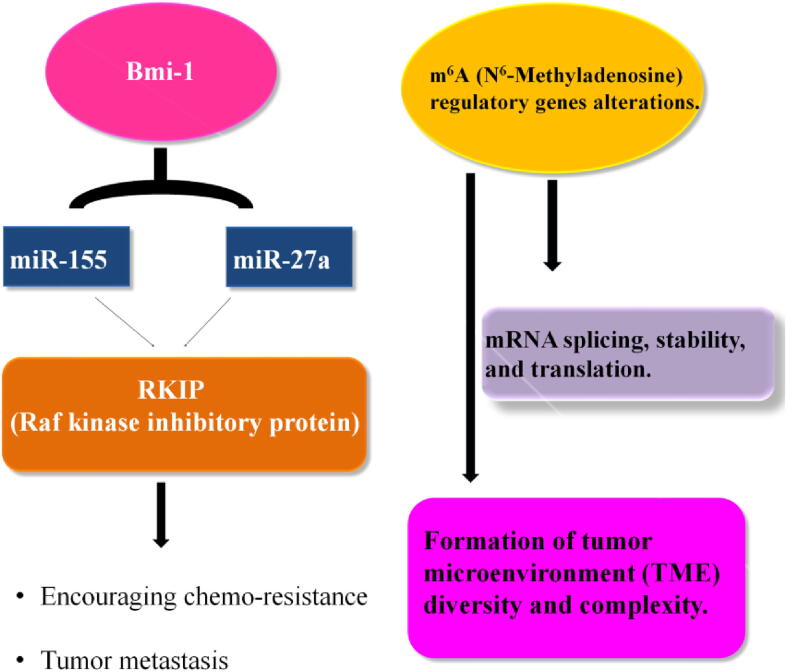
Fig. 2.
Role of miRNA involved in cancer treatment (Li et al., 2020, Zhang et al., 2020).
Fig. 3.
Chemotherapeutic drug transportation, metabolism and their mechanistic action (Kim et al., 2011, Jeon et al., 2011).
Helicobacter pylori (H. pylori)infection in the stomach remains a main risk factor that promotes the development of GC through induction of chronic gastritis, and genetic mutations (Zhang et al., 2020). H. pylori is diagnosed as a first group carcinogen according to the IARC (International Agency for Research on Cancer) and is mainly associated with non-cardia gastric carcinoma (Inoue, 2017). About 20% of patients undergoing surgery have the chance of developing surgical site infections (SSI) by H. pylori which is regarded as a common agent of nosocomial infections (Kim et al., 2011). Apart from this, the other risk factors contributing to the cause are smoking, dietary factors including obesity, contaminated air, lifestyle modifications, and stress (Oh et al., 2009).
The pathogenic H. pylori and gastric cancers has been mentioned in numerous studies, and those have proven that H. pylori-related carcinogenesis is commonly because of the gastric inflammatory reaction and ensuing oxidative stress (Inoue, 2017). It has been shown among the H. pylori contamination, the cases of gastric cancers is highest among the CagA (cytotoxin-associated gene A)-positive group (Park et al., 2010). Studies shown that the pathway through which H. pylori causes gastric cancer includes the enhancement of chronic inflammation to the mucosal layer of stomach, and during progression through pre-cancerous lesions (dysplasia, metaplasia and atrophic gastritis), H. pylori is known to cause mutation of genes (Park et al., 2010). It has been hypothesized that a comparable mechanism is associated with the other cancers such as colorectal cancer from adenoma to adenocarcinoma; particularly, the mechanism of inactivating the tumor suppressor genes, mismatch repair of DNA occurs and activation of oncogenes ensuing in the incline of gastric cancer (Park et al., 2010).
GC is a well-known disease that stipulates continued heed and research for prevention (Eom et al., 2016). At the primary stage, the specific diagnosis of GC is strenuous as there are no signs and symptoms (Park et al., 2019). The rate of survival from GC is roughly 25% after preliminary diagnosis. There have been many latest diagnostic modalities and therapeutics for the disease (Song et al., 2016). The cases of SSI in gastric cancer surgery were found to be from 5% to 20% based on the number of patients, and operating methods. Approximately, 3 to 6 Antimicrobial prophylaxis (AMP) can successfully put a stop to surgical site infections (SSI) in gastric cancer surgery (J.H. et al., 2014). Chemotherapy and radiotherapy are exceedingly efficacious in treating different types of cancer (Seol et al., 2016). These modern therapeutics also accompany adverse effects with them leading to damages of normal and healthy tissues, prompting programmed cell death and altering normal cellular functions (Kwon et al., 2014).
Hence, these advanced therapeutics with several side-effects may be considered as one of the major reasons for the rise in death rates. The average survival time of the metastatic cancer report is around one year (Sohn et al., 2017). Hence, prior diagnosis and therapy are necessary to increase the survival rates in gastric cancer, since a complex set of causes attribute to the tumor development. Thereby highlighting the attention towards therapeutic strategies to control the sequels of GC during disease progression, therapy, and recovery phases are important (Tokioka et al., 2012).
2. Present day standard therapeutics in gastric cancer
Many advanced treatment methods are put forth for gastric cancer, which includes molecular targeted therapeutics, immunotherapy, and post-operative adjuvant chemotherapy.
2.1. Role of immune-checkpoint inhibitors in GC therapeutics
Immunological checkpoint inhibitions are one of the successful therapeutic approaches during malignancies (Lee et al., 2020). Recent reports revealed the positive effects of immunotherapy for patients suffering from cancer where therapies associated with immune checkpoint inhibitors like programmed death-ligand 1 (PD-L1) inhibitor or anti-programmed death-1 receptor (PD-1) (Park et al., 2019) (see Fig. 1). Studies on clinical trial have concluded that the immune surrounding microenvironment of tumor cells usually correspond with the reaction rate to anti‐PD‐1 or PD‐L1 therapeutics. Recognition of biomarkers to anticipate frequency risk is important to improve adjuvant treatment approach in stage two/three of GC (H. Kim et al., 2018a).
In hepatocellular carcinoma (HCC), PD-1(programmed cell death protein-1)/PD-L1 (programmed death ligand-1) pathways have been inhibited by immune checkpoint inhibitors (ICIs). However, only a small group of GC patients supported these therapeutic techniques, indicating the need for significant biomarkers identification to enhance clinical benefits (Ho et al., 2020). Arresting the communication between PD‐1 protein with its ligands such as PD‐L1, and PD‐L2 may lead to an effective antitumor response and improve clinical outcomes in patients (Ishikawa et al., 2018) (see Fig. 1). Increased synthesis of PD-1 protein and defective CD8 + T cell functions are interlinked which results in tumor immune escape (Shen et al., 2020a, Shen et al., 2020b). The high concentration of transforming growth factor-β1 (TGF-β1) in cancerous cells has a beneficial correlation with PD-1 protein, cytotoxic T cells (CD8 + T) cell incursion, and gastric cancer cells derived TGF-β1 has an influence on PD-1 reflection and CD8 + T cells through the Smad3 signalling pathway under controlled conditions. Renovating the role of cytotoxic T cells by combining PD-1 protein and TGF-β1 blockage may have a high influence on future GC immunotherapeutic (Shen et al., 2020a, Shen et al., 2020b).
Despite several attainments in immune-checkpoint-blockade (ICB) to cure cancer, there are a few effective anticipating biomarkers in gastric cancer (Lee et al., 2020). The functioning of two biomarkers of CNA (copy-number alterations) burden and TMB (tumor mutational burden) may help DCB (durable clinical benefit) patients grouping, thus giving a logical option for patients medicated with immune checkpoint blockade (Lee et al., 2020). However, forecasting tumor reaction to PD‐1 blockade still remains complicated (Ishikawa et al., 2018).
Cancer treatment based on chimeric antigen receptor (CAR) T cells have shown significant results in the therapeutic ability of CAR-T cells intending ICAM-1 in preclinical patterns of fundamental and intraperitoneal metastases of GC (Jung et al., 2020). The combined therapy of paclitaxel conversely, protecting CAR-T cells along experimental IL-12 is known to increase the therapeutic result of CAR-T cells (Jung et al., 2020).
Metastasis in lymph node is one of the best foreboding indicators in non-distant metastatic gastric cancer (E. W. Lee et al., 2016a). NPTXR controls the malignant nature of gastric cancer cells in controlled and natural conditions (Kanda et al., 2020). The innovative mechanism of action of antibodies targeting PD‐1 and PD‐L1 is concorded with particular adverse events such as immune‐related thyroiditis, pneumonitis, or colitis, etc., including novel motifs of responses like intellectual and reliable responses, pseudo progression (PSPD), and hyper progressive disease (HP) (Kawazoe et al., 2017). PSPD emerges most often in patients having a primary increase in target lesions, indicating that PSPD must be examined only in these patients (Kawazoe et al., 2017).
NPTXR silencing is known to enhance the caspase-mediated cell death pathways and enervate gastric cancer cell proliferation, migration, stem cell features, adhesion, invasion, cell cycle, and 5-fluorouracil resistance under laboratory conditions, and also down-regulation of malignancy of gastric cancer cells in natural conditions (Kanda et al., 2020). Gastric cancer patient showing PRKDC/ DNA-PK(PRKDC, codes for the catalytic subunit of DNA dependent protein kinase)mutations were greatly correlated with microsatellite instability (MSI) immense status and PRKDC increases the capacity of the anti-apoptotic protein one pathway monoclonal antibody (mAB) in the CT26 animal model. The co-stimulatory receptor 4-1BB[cluster of differentiation 137(CD-137), Tumor necrosis factor receptor superfamily member 9 (TNFRSF9)] having a crucial enduring effect on T cell immune response is detected as a target for therapeutics (Trüb et al., 2020).
Activating programmed cell death in cisplatin mediated cells through hindering ERK and Akt pathways leads to the USP14 simulated GC cells to cisplatin. This approach of integrating USP14 impedance with cisplatin‐mediated therapeutics enhanced the result of GC patients (Fu et al., 2018). The expression of four genes such as IL-12 (interleukin), p35,granulocyte–macrophage colony-stimulating factor (GM-CSF), IL-12p40, and relaxin (RLX) regulated by a single oncolytic Ad (adenovirus) vector could enhance alteration of both physical and immunological characteristics of the tumor niches to overcome the key constrains of antibody-based treatments that have emerged in recent clinical trials (Jung et al., 2020). Combined therapeutics with FAP (fibroblast activation protein)-4-1BBL and T cell receptor activation by either anti-CD3 (cluster of differentiation 3) or T cell bispecific antibodies remarkably stimulated the tumor-infiltrating lymphocytes (TIL) and its effector functions, comprising proliferation of T cells, discharge of pro-inflammatory cytokines (Trüb et al., 2020). Conspicuously, co-stimulation with FAP-4-1BBL resulted in the production of interleukin (IL) − 13 which is correlated with apoptosis of tumor cells, which is cytokine-mediated and it is partly dependent on signal transducer and activator of transcription 6 (STAT6) phosphorylation and IL-13 alpha 1 or 2 receptors (Trüb et al., 2020).
Oncogram is considered as a patient-specific tool that appraises clinical factors including some biological characteristics and also treatment-specific factors known as virus arming (Hemminki et al., 2018). Oncogram is one of the pioneers comprehensive attempts to diagnose the patients more feasibly to encourage from oncolytic adenoviral therapy where it involves immunological laboratory parameters evaluated in peripheral white blood cells, interleukin-8 (IL-8), lymphocyte-to-neutrophil ratio, anti-viral neutralizing antibody status, the physiognomy of the patient such as performance status, gender and tumor characteristics like tumor load, histological tumor category, the site of metastases, and oncolytic virus-specific attributes including arming the virus (Hemminki et al., 2018). By estimating cytokines and relevant amounts of immune cells in blood serum, the diagnosis of patients with metastatic gastric cancer (mGC) can be determined (Park et al., 2019). Gastric cancer patients responding to immune checkpoint blocker (ICB) therapy showed a remarkably higher one-year furtherance free and generally more survival rate than non-responders (Lee et al., 2020).
2.2. m-RNA and mi-RNA involved in gastric cancer therapy
MicroRNAs (miRNAs) have a major role in modulating gene expression during gastric cancer metastasis (Cheng et al., 2020). MicroRNAs are known to have a major impact on post-transcriptional modifications of messenger RNA and influence on tumor formation in humans (Chong et al., 2017). The variation in miRNAs expression may result in tumor suppression or oncogene expression and give rise to a sequence of fatal pathological pathways in GC (Cheng et al., 2020). Malfunctioning of microRNAs (miRNAs) leads to cancer by abnormal proliferation of cells, apoptosis, and differentiation (S. W. Lee et al., 2016b). Activation of both HOXA10 (Homeobox A10) and miR-196b indicated a weak prognosis of GC in a subset of patients. Interpretation of the molecular function of HOXA10 and miR-196b is justified (Lim et al., 2013). The regulatory system of miR-29 s may influence the advancements in the pathological conditions as well as growth patterns of GC by controlling the expression of the COL4A1 gene, whose inhibition gradually down-regulates the invasion and migration of GC cells (Lim et al., 2013). miR-155 plays a key role in angiogenesis where it can improve the formation of new vessels in vitro by downregulating the secretion of Forkhead box O3 (FOXO3a) protein, that results in the progress of GC therapeutics (Zhou et al., 2019). Thereby, miR-155 has become a promising biomarker for the identification of angiogenesis processes and migration in GC, and thus could be an important targeting molecule for anti-angiogenesis therapy (Zhou et al., 2019).
Bmi-1(B cell-specific Moloney murine leukemia) was known to influence the function of miR-155 and miR-27a thereby encouraging chemoresistance and tumor metastasis by targeting RKIP (Raf kinase inhibitory protein) in GC (Li et al., 2020) (see Fig. 2). Studies also revealed that exosomal miR-522(exo-miR-522) was synthesized by CAFs (Cancer-associated fibroblasts) to hinder the ferroptosis in cancerous tissue by effecting ALOX15 (arachidonate lipoxygenase15) expression and inhibiting the assemblage of lipid-ROS (Zhang et al., 2020) (see Fig. 2). Co-activation of miR-375 and miR-196b-5p remarkably correspond with GC. These miRNAs thereby play their role as a diagnostic biomarkers of gastric cancer (S. W. Lee et al., 2016b). The cellular pathway, involving heterogeneous nuclear ribonucleoprotein A1 (hnRNPA1), ubiquitin-specific protease 7 (USP7), ALOX15, and exo-miR-522, provides the latest mechanism of gained chemo-resistance in gastric cancer (Zhang et al., 2020). The alleviated levels of Plasminogen activator inhibitor-1 (PAI-1) may act as an anticipating marker of initial stages in gastric cancer and regulation of the urokinase plasminogen activator (uPA) system comprising PAI-1 positively correlated with peritoneal metastasis of GC (Suh et al., 2015). These studies state that PAI-1 RNAi decreases gastric peritoneal metastasis and inhibits tumor progression (Suh et al., 2015).
Modifications in m6A (N6-Methyladenosine) regulatory genes have a high impact on the pathogenesis of different human diseases (Yue et al., 2019). m6A alterations have been involved in various biological processes and play a crucial role by regulating the mRNA splicing, stability, and translation (Shen et al., 2020a, Shen et al., 2020b). The m6A alterations are involved in the formation of tumor microenvironment (TME) diversity and complexity. Assessing the m6A modification motifs of the tumor will help to increase the awareness of TME infiltration characterization (Zhang et al., 2020). Dysregulating the m6A, its pathways in tumor epithelial-mesenchyme transition (EMT), the significant functions of m6A alterations in GC and METTL3/E-cadherin/ZMYM1signaling is an effective therapy which targets anti-metastatic pathway in GC (Yue et al., 2019). However, there is an advanced approach that unified the state of the art sequential windowed acquisition of all theoretical fragment ion (SWATH) mass spectra (MS) along with multi dataset combined assay for the diagnosis of first stage gastric cancer plasma biomarker and CHI3L1 gene is identified as a promising biomarker for patients at early gastric cancer (Min et al., 2020).
2.3. Chemotherapeutic drugs and their combination
Systemic chemotherapy is suggested as the standard treatment for patients having advanced gastric cancer (Kim et al., 2011) (see Fig. 3).The spreading of gastric cancer is often due to the exaggerated expression of wild-type p21 proteins and the mutated Ras proteins (Wang et al., 2018). Patients with advanced gastric cancer and distant metastasis continue to be inoperable, and known to exhibit median survival despite chemotherapeutics administration (Kim et al., 2011). Abnormal expressions of ERBB proteins are oncogenic (Choi et al., 2012). Adenovirus KGHV500 has known to cease migration, proliferation, invasiveness of SGC7901 cells, and instigate cellular apoptosis under controlled conditions (Wang et al., 2018). CD44 is a prime adhesion molecule and cell surface receptor for hyaluronic acid which acts as a moral biomarker for prognosis forecasting and biomarker-driven therapies only for early gastric cancer (Go et al., 2016). The ErbB family of receptors include many growth factors among which human epidermal growth factor receptor 2 (HER2) and epidermal growth factor receptor (EGFR) are the main therapeutic targets for lung, breast, and gastric cancers (T. M. Kim et al., 2018b). Both EGFR and HER2 comprising tiny molecule kinase inhibitors like gefitinib, erlotinib, and lapatinib are skillfully targeted for cancer treatments (Choi et al., 2012). The antitumor response to oncolytic recombinant adenovirus was accomplished by cytokine-induced killer (CIK) cells on human gastric cells (SGC7901 cells) which elevated the expression of wild-type Ras (Wang et al., 2018). The up-regulation of HER2 is well known to result in roughly 15% to 20% of gastric cancer patients (Yi et al., 2016). Lapatinib is a principle drug used to treat human epidermal growth factor receptor 2, trastuzumab-resistant (Park et al., 2018). Aspirin is one of the broadly accepted anti-cancerous drugs. The anti-cancer nature of aspirin is majorly linked to the inhibition of the cyclooxygenase (COX) pathway correlating to the anti-inflammatory mechanism of aspirin, thereby, it is been reported that low-dosage aspirin helps in reducing the risk of gastric cancer (Y. Il Kim et al., 2016a).
Asirinotecan and docetaxel are considered as second-line chemotherapeutics for gastric cancer (Jeon et al., 2011) (see Fig. 3). The most frequent negative response to drug-related treatment include stomatitis, diarrhoea, pruritus, rash, and anorexia. Poziotinib drug was recommended in patients having advanced solid tumors (T. M. Kim et al., 2018b).
Platinum-based chemotherapeutics are generally preferred as first-line chemotherapeutics for advanced gastric cancer (Park et al., 2019). Studies determined the efficiency and toxicity of the FOLFIRI procedure as a second-line therapeutic method when the altered FOLFOX-4 mechanism was unsuccessful (Jeon et al., 2011). The 5-FU based regimen associated with cisplatinum has shown the least significant superior survival time than a single 5-FU regimen but both had improved the median survival in contrast to surgery (Yamada et al., 2012). Rescued docetaxel mediated chemotherapy is a possible therapeutic choice for advanced gastric cancer (AGC) patients exhibiting better performance status (PS), whereas chemotherapy for patients showing low PS must be undertaken with attention for those who are unsuccessful earlier with oxaliplatin and irinotecan-based regimens (Lee et al., 2012). Poziotinib is a drug that exhibits a remarkable activity contrary to EGFR-mutant and HER2-augmented cancers (T. M. Kim et al., 2018b). OXO1 is a crucial linker between MET signalling pathways and HER2 via adverse crosstalks and known to perform (Kim, 2014) important regulations of the gained lapatinib resistance in HER2 positive gastric cancerous cells (Park et al., 2018). The advised treatment for metastatic gastric cancer (MGC) include fluoropyrimidines, platinum, anthracyclines, taxanes, irinotecan as first-line chemotherapeutics and no standard treatments are reported for second-line therapy (Carter et al., 2017). Fork head transcription factors of the O class (FOXOs) in mammals which is categorized into 4 classes: FOXO4, FOXO1, FOXO6, and FOXO3 are not much expressed in gastric cancerous cells (Zhou et al., 2019). Downregulation of angiogenesis by the retrieval of FOXO1 function and significant inhibition of vascular endothelial growth factor (VEGF) and hypoxia-inducible factor-1α(HIF-1α), maybe a fascinating approach for gastric cancer therapeutics (S. Y. Kim et al., 2016b).
2.4. Cancer therapeutics related to age, sex, disease, etc
Various studies discovered that health behaviours like smoking, alcohol addiction, and inactive motifs of cancer survivors were significantly associated with elevated morbidity risks. The impact of these health behaviours such as age, education, marital status, sex, self-rated health status, employment state, diagnosis frequency, body mass index (BMI), the heredity of cancer, and comorbid chronic disease on GC survivors, are studied (Y. Il Kim et al., 2016a). The comorbid chronic disease could be any of the diseases such as hypertension, stroke dyslipidaemia, diabetes, myocardial infarction or heart arrest, colon polyp, transient ischemic attacks, chronic liver disease or liver cirrhosis, fatty liver cholecystitis, thyroid disease, osteoporosis, and arthritis (Y. Il Kim et al., 2016a). Epidemiological investigations have reported the affiliation between gastric cancer risk and increased salt intake. However, the association between salt intake and gastric cancer screening in the local population has scarcely been evaluated. Individuals preferring excess salt exhibited suboptimal gastric cancer screening adherence in contrast to those who prefer low salt. Hence, these results focus on the demand for the best conveyance message of awareness to alter risk perspicacity about the practice of gastric cancer screening (Shin et al., 2016). Studies reported the significant decrease in gastric and lung cancer cases in the urban region whereas the cases continue to rise in a countryside. However, an upcoming confirmation study is necessary to decide the reasons for the variation in cancer frequency by population size (Song et al., 2016).
The global GC screening program approved the approximate interval for proximal gastric screening in both males and females which is a two-year interval, 24 months. The death rate concordant to decline in gastric cancer as a result of the distribution of gastric cancer screening programs also progressed in surgical techniques, chemotherapy, and radiotherapy (Bae et al., 2015). The studies reported the gastric cancer risk correlated with body mass index (BMI) levels in a U-shaped manner, particularly in populations not infected by H. pylori (Jang et al., 2019). The studies have revealed that the cancer surgical death rate differs highly which can be illustrated fortuitously in a treated subset of patients and that accordingly; this variation is the main reason for increased and futile mortality (Lipitz-Snyderman et al., 2020). The quality measurement may be beneficial for the patients to decide value‐based compensatory programs, policy evaluation, and quality battering initiatives with the latest goal of developing patient outcomes (Lipitz-Snyderman et al., 2020). As the generations change, clinicians are elevating being challenged with the difficulties in treating aged patients, as the number of old aged gastric cancer patients has been rising (J.I. et al., 2019). Various results concluded that aged patients believed to receive less therapeutics for gastric cancer than youths (Sohn et al., 2017). This inclusive evaluation of the disparity and racial gastric cancer cases in The United States authenticated a tedious decline in such disparities along with time, mainly as estimated on the absolute scale. Thus, the decline is primarily compelled by the reduction in disparities in non-cardia GC cases and can owe to the least predominance of H. pylori infection accelerated in American and Asian populations (Yao et al., 2019).
3. Conclusion
Despite the significant fall in the gastric cancer cases over the last few decades, there is still serious room for progression concerning prevention, early prognosis, mediation, and therapies which increase the survival rate in patients. Advanced improvements in sequencing techniques are tending to revolutionize our acceptance of disease biology in the coming decades. It's promising that several recent biomarkers will give an exclusively new choice for personalized therapy, which would appreciate significant therapeutic enhancements, with extraordinary potency and tolerable unfavourable effects.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgement
CRA is thankful to the Central University of Punjab and Indian Academy of Sciences, Bangalore for organizing the Virtual Internship with Science Leader (VISL) program, the outcome which is this review. SKP, SB and SR thank the JSS Academy of Higher Education and Research (JSSAHER) for the infrastructure and encouragement provided. This research was partially supported by Chiang Mai University.
Author Contributions
All authors contributed equally.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Ratchadawan Cheewangkoon, Email: ratchadawan.c@cmu.ac.th.
Shashanka K. Prasad, Email: shashankaprasad@jssuni.edu.in.
References
- Bae J.M., Shin S.Y., Kim E.H. Optimal interval for repeated gastric cancer screening in normal-risk healthy Korean adults: A retrospective cohort study. Cancer Res. Treat. 2015 doi: 10.4143/crt.2014.098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter G.C., Kaltenboeck A., Ivanova J., Liepa A.M., Roman A.S., Koh M., Rajan N., Cheng R., Birnbaum H.G., Kim J.S., Bang Y.J. Real-world treatment patterns among patients with advanced gastric cancer in South Korea. Cancer Res. Treat. 2017 doi: 10.4143/crt.2016.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, J., Zhuo, H., Wang, L., Zheng, W., Chen, X., Hou, J., Zhao, J., Cai, J., 2020. Identification of the Combinatorial Effect of miRNA Family Regulatory Network in Different Growth Patterns of GC. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2020.03.012. [DOI] [PMC free article] [PubMed]
- Choi B.K., Fan X., Deng H., Zhang N., An Z. ERBB3 (HER3) is a key sensor in the regulation of ERBB-mediated signaling in both low and high ERBB2 (HER2) expressing cancer cells. Cancer Med. 2012 doi: 10.1002/cam4.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong, G.O., Jeon, H.S., Han, H.S., Son, J.W., Lee, Y.H., Hong, D.G., Park, H.J., Lee, Y.S., Cho, Y.L., 2017. Overexpression of microRNA-196b accelerates invasiveness of cancer cells in recurrent epithelial ovarian cancer through regulation of Homeobox A9. Cancer Genomics and Proteomics. https://doi.org/10.21873/cgp.20026. [DOI] [PMC free article] [PubMed]
- Eom B.W., Kim Y.W., Nam B.H., Ryu K.W., Jeong H.Y., Park Y.K., Lee Y.J., Yang H.K., Yu W., Yook J.H., Song G.A., Youn S.J., Kim H.U., Noh S.H., Park S.B., Yang D.H., Kim S. The Korean gastric cancer cohort study: Study protocol and brief results of a large-scale prospective cohort study. J. Gastric Cancer. 2016 doi: 10.5230/jgc.2016.16.3.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Ma G., Liu G., Li B., Li H., Hao X., Liu L. USP14 as a novel prognostic marker promotes cisplatin resistance via Akt/ERK signaling pathways in gastric cancer. Cancer Med. 2018 doi: 10.1002/cam4.1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Go S. Il, Ko G.H., Lee W.S., Kim R.B., Lee J.H., Jeong S.H., Lee Y.J., Hong S.C., Ha W.S. CD44 variant 9 serves as a poor prognostic marker in early gastric cancer, but not in advanced gastric cancer. Cancer Res. Treat. 2016 doi: 10.4143/crt.2014.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemminki, O., Oksanen, M., Taipale, K., Liikanen, I., Koski, A., Joensuu, T., Kanerva, A., Hemminki, A., 2018. Oncograms Visualize Factors Influencing Long-Term Survival of Cancer Patients Treated with Adenoviral Oncolytic Immunotherapy. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2018.04.003.. [DOI] [PMC free article] [PubMed]
- Ho W.J., Danilova L., Lim S.J., Verma R., Xavier S., Leatherman J.M., Sztein M.B., Fertig E.J., Wang H., Jaffee E., Yarchoan M. Viral status, immune microenvironment and immunological response to checkpoint inhibitors in hepatocellular carcinoma. J. Immunother. Cancer. 2020 doi: 10.1136/jitc-2019-000394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue M. Changing epidemiology of Helicobacter pylori in Japan. Gastric Cancer. 2017 doi: 10.1007/s10120-016-0658-5. [DOI] [PubMed] [Google Scholar]
- Ishikawa E., Tanaka T., Shimada K., Kohno K., Satou A., Eladl A.E., Sakakibara A., Furukawa K., Funasaka K., Miyahara R., Nakamura M., Goto H., Nakamura S., Kato S., Hirooka Y. A prognostic model, including the EBV status of tumor cells, for primary gastric diffuse large B-cell lymphoma in the rituximab era. Cancer Med. 2018 doi: 10.1002/cam4.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J.H., Jeong O., Ryu S.Y., Jung M.R., Park Y.K. Efficacy of single-dose antimicrobial prophylaxis for preventing surgical site infection in radical gastrectomy for gastric carcinoma. J. Gastric Cancer. 2014 doi: 10.5230/jgc.2014.14.3.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J.I., Lim D.H., Lee J., Kang W.K., Park S.H., Park J.O., Park Y.S., Lim H.Y., Kim S.T., Lee S.J., Kim S., Sohn T.S., Lee J.H., An J.Y., Choi M.G., Bae J.M., Yoo H., Kim K. Comparison of the 7th and the 8th AJCC Staging System for Non-metastatic D2-Resected Lymph Node-Positive Gastric Cancer Treated with Different Adjuvant Protocols. Cancer Res. Treat. 2019 doi: 10.4143/crt.2018.401. LK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang J., Cho E.J., Hwang Y., Weiderpass E., Ahn C., Choi J., Chang S.H., Shin H.R., Lim M.K., Yoo K.Y., Park S.K. Association between body mass index and gastric cancer risk according to effect modification by helicobacter pylori infection. Cancer Res. Treat. 2019 doi: 10.4143/crt.2018.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon E.K., Hong S.H., Kim T.H., Jung S.E., Park J.C., Won H.S., Ko Y.H., Rho S.Y., Hong Y.S. Modified FOLFIRI as second-line chemotherapy after failure of modified FOLFOX-4 in advanced gastric cancer. Cancer Res. Treat. 2011 doi: 10.4143/crt.2011.43.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung, M., Yang, Y., McCloskey, J.E., Zaman, M., Vedvyas, Y., Zhang, X., Stefanova, D., Gray, K.D., Min, I.M., Zarnegar, R., Choi, Y.Y., Cheong, J.-H., Noh, S.H., Rha, S.Y., Chung, H.C., Jin, M.M., 2020. Chimeric Antigen Receptor T Cell Therapy Targeting ICAM-1 in Gastric Cancer. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2020.08.009. [DOI] [PMC free article] [PubMed]
- Kanda, M., Shimizu, D., Sawaki, K., Nakamura, S., Umeda, S., Miwa, T., Tanaka, H., Tanaka, C., Hayashi, M., Iguchi, Y., Yamada, S., Katsuno, M., Kodera, Y., 2020. Therapeutic monoclonal antibody targeting of neuronal pentraxin receptor to control metastasis in gastric cancer. Mol. Cancer. https://doi.org/10.1186/s12943-020-01251-0. [DOI] [PMC free article] [PubMed]
- Kawazoe A., Kuwata T., Kuboki Y., Shitara K., Nagatsuma A.K., Aizawa M., Yoshino T., Doi T., Ohtsu A., Ochiai A. Clinicopathological features of programmed death ligand 1 expression with tumor-infiltrating lymphocyte, mismatch repair, and Epstein-Barr virus status in a large cohort of gastric cancer patients. Gastric Cancer. 2017 doi: 10.1007/s10120-016-0631-3. [DOI] [PubMed] [Google Scholar]
- Kim H., Hwang Y., Sung H., Jang J., Ahn C., Kim S.G., Yoo K.Y., Park S.K. Effectiveness of gastric cancer screening on gastric cancer incidence and mortality in a community-based prospective cohort. Cancer Res. Treat. 2018 doi: 10.4143/crt.2017.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Han Jo, Eun J.Y., Jeon Y.W., Yun J., Kim K.H., Kim S.H., Kim Hyun Jung, Lee S.C., Bae S.B., Kim C.K., Lee N.S., Lee K.T., Park S.K., Won J.H., Hong D.S., Park H.S. Efficacy and safety of oxaliplatin, 5-fluorouracil, and folinic acid combination chemotherapy as first-line treatment in metastatic or recurrent gastric cancer. Cancer Res. Treat. 2011 doi: 10.4143/crt.2011.43.3.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y. Il, Kim S.Y., Kim J.H., Lee J.H., Kim Y.W., Ryu K.W., Park J.H., Choi I.J. Long-term low-dose aspirin use reduces gastric cancer incidence: A nationwide cohort study. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.W. The result of conversion surgery in gastric cancer patients with peritoneal seeding. J. Gastric Cancer. 2014 doi: 10.5230/jgc.2014.14.4.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.Y., Ko Y.S., Park J., Choi Y., Park J.W., Kim Y., Pyo J.S., Yoo Y.B., Lee J.S., Lee B.L. Forkhead transcription factor FOXO1 inhibits angiogenesis in gastric cancer in relation to SIRT1. Cancer Res. Treat. 2016 doi: 10.4143/crt.2014.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T.M., Lee K.W., Oh D.Y., Lee J.S., Im S.A., Kim D.W., Han S.W., Kim Y.J., Kim T.Y., Kim J.H., Han H., Kim W.H., Bang Y.J. Phase 1 studies of poziotinib, an irreversible pan-her tyrosine kinase inhibitor in patients with advanced solid tumors. Cancer Res. Treat. 2018 doi: 10.4143/crt.2017.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon O.K., Chung H.Y., Yu W. Early postoperative intraperitoneal chemotherapy for macroscopically serosa-invading gastric cancer patients. Cancer Res. Treat. 2014 doi: 10.4143/crt.2014.46.3.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.W., Lee W.Y., Koo H.S. Prognostic factors for node-negative advanced gastric cancer after curative gastrectomy. J. Gastric Cancer. 2016 doi: 10.5230/jgc.2016.16.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Chang J.S., Roh M.R., Jung M., Lee C.K., Oh B.H., Chung K.Y., Koom W.S., Shin S.J. Clinical Outcomes of Immune Checkpoint Blocker Therapy for Malignant Melanoma in Korean Patients: Potential Clinical Implications for a Combination Strategy Involving Radiotherapy. Cancer Res. Treat. 2020 doi: 10.4143/crt.2019.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M.J., Hwang I.G., Jang J.S., Choi J.H., Park B.B., Chang M.H., Kim S.T., Park S.H., Kang M.H., Kang J.H. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res. Treat. 2012 doi: 10.4143/crt.2012.44.4.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S.W., Park K.C., Kim J.G., Moon S.J., Kang S.B., Lee D.S., Sul H.J., Ji J.S., Jeong H.Y. Dysregulation of microRNA-196b-5p and microRNA-375 in gastric cancer. J. Gastric Cancer. 2016 doi: 10.5230/jgc.2016.16.4.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., Li, Y., Tian, Z., Tian, Z., Tan, Y., Tan, Y., Lian, G., Lian, G., Chen, Shangxiang, Chen, Shangxiang, Chen, Shaojie, Chen, Shaojie, Li, J., Li, J., Li, X., Li, X., Huang, K., Huang, K., Chen, Y., Chen, Y., 2020. Bmi-1-induced miR-27a and miR-155 promote tumor metastasis and chemoresistance by targeting RKIP in gastric cancer. Mol. Cancer. https://doi.org/10.1186/s12943-020-01229-y. [DOI] [PMC free article] [PubMed]
- Lim, J.Y., Yoon, S.O., Seol, S.Y., Hong, S.W., Kim, J.W., Choi, S.H., Lee, J.S., Cho, J.Y., 2013. Overexpression of miR-196b and HOXA10 characterize a poor-prognosis gastric cancer subtype. World J. Gastroenterol. https://doi.org/10.3748/wjg.v19.i41.7078. [DOI] [PMC free article] [PubMed]
- Lipitz-Snyderman A., Lavery J.A., Bach P.B., Li D.G., Yang A., Strong V.E., Russo A., Panageas K.S. Assessment of variation in 30-day mortality following cancer surgeries among older adults across US hospitals. Cancer Med. 2020 doi: 10.1002/cam4.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv Y., Zhao Y., Wang X., Chen N., Mao F., Teng Y., Wang T., Peng L., Zhang J., Cheng P., Liu Y., Kong H., Chen W., Hao C., Han B., Ma Q., Zou Q., Chen J., Zhuang Y. Increased intratumoral mast cells foster immune suppression and gastric cancer progression through TNF-?-PD-L1 pathway. J. Immunother. Cancer. 2019 doi: 10.1186/s40425-019-0530-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, L., Zhu, S., Wei, R., Zhao, Y., Liu, S., Li, P., Zhang, S., 2020. Integrating SWATH-MS Proteomics and Transcriptome Analysis Identifies CHI3L1 as a Plasma Biomarker for Early Gastric Cancer. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2020.03.020. [DOI] [PMC free article] [PubMed]
- Oh D.-Y., Choi K.S., Shin H.-R., Bang Y.-J. Public Awareness of Gastric Cancer Risk Factors and Disease Screening in a High Risk Region: A Population-Based Study. Cancer Res. Treat. 2009 doi: 10.4143/crt.2009.41.2.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D.W., Lee K.J., Jin S.H., Lee J.H., Min J.S., Park S.H., Yu H.J., Bang H.Y., Lee J.I. Phenotypic differences of gastric cancer according to the Helicobacter pylori infection in Korean patients. J. Gastric Cancer. 2010 doi: 10.5230/jgc.2010.10.4.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H.S., Kwon W.S., Park S., Jo E., Lim S.J., Lee C.K., Lee J.B., Jung M., Kim H.S., Beom S.H., Park J.Y., Kim T.S., Chung H.C., Rha S.Y. Comprehensive immune profiling and immune-monitoring using body fluid of patients with metastatic gastric cancer. J. Immunother. Cancer. 2019 doi: 10.1186/s40425-019-0708-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J., Choi Y., Ko Y.S., Kim Y., Pyo J.S., Jang B.G., Kim M.A., Lee J.S., Chang M.S., Park J.W., Lee B.L. FOXO1 suppression is a determinant of acquired lapatinib-resistance in HER2-positive gastric cancer cells through MET upregulation. Cancer Res. Treat. 2018 doi: 10.4143/crt.2016.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seol S.Y., Kim C., Lim J.Y., Yoon S.O., Hong S.W., Kim J.W., Choi S.H., Cho J.Y. Overexpression of endoplasmic reticulum oxidoreductin 1-α (ERO1L) is associated with poor prognosis of gastric cancer. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, C., Xuan, B., Yan, T., Ma, Y., Xu, P., Tian, X., Zhang, X., Cao, Y., Ma, D., Zhu, X., Zhang, Y., Fang, J Y., Chen, H., Hong, J., 2020. m6A-dependent glyocolysis enhancers colorectal cancer progression. Mol. Cancer. https://doi.org/10.1186/s12943-020-01190-w. [DOI] [PMC free article] [PubMed]
- Shen Y., Teng Y., Lv Y., Zhao Y., Qiu Y., Chen W., Wang L., Wang Y., Mao F., Cheng P., Ma D., Zhuang Y., Zou Q., Peng L. PD-1 does not mark tumor-infiltrating CD8+ T cell dysfunction in human gastric cancer. J. Immunother. Cancer. 2020 doi: 10.1136/jitc-2019-000422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin J.Y., Kim J., Choi K.S., Suh M., Park B., Jun J.K. Relationship between salt preference and gastric cancer screening: An analysis of a nationwide survey in Korea. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sohn I.W., Jung D.H., Kim J.H., Chung H.S., Park J.C., Shin S.K., Lee S.K., Lee Y.C. Analysis of the clinicopathological characteristics of gastric cancer in extremely old patients. Cancer Res. Treat. 2017 doi: 10.4143/crt.2016.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song H.N., Go S. Il, Lee W.S., Kim Y., Choi H.J., Lee U.S., Kang M.H., Lee G.W., Kim H.G., Kang J.H., Kang Y.S., Lee J.H., Jung J.M., Hong S.C. Population-based regional cancer incidence in Korea: Comparison between urban and rural areas. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh Y.S., Yu J., Kim B.C., Choi B., Han T.S., Ahn H.S., Kong S.H., Lee H.J., Kim W.H., Yang H.K. Overexpression of plasminogen activator inhibitor-1 in advanced gastric cancer with aggressive lymph node metastasis. Cancer Res. Treat. 2015 doi: 10.4143/crt.2014.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokioka S., Umegaki E., Murano M., Takeuchi N., Takeuchi T., Kawakami K., Yoda Y., Kojima Y., Higuchi K. Utility and problems of endoscopic submucosal dissection for early gastric cancer in elderly patients. J. Gastroenterol. Hepatol. 2012 doi: 10.1111/j.1440-1746.2012.07075.x. [DOI] [PubMed] [Google Scholar]
- Trüb M., Uhlenbrock F., Claus C., Herzig P., Thelen M., Karanikas V., Bacac M., Amann M., Albrecht R., Ferrara-Koller C., Thommen D., Rothschield S., Savic Prince S., Mertz K.D., Cathomas G., Rosenberg R., Heinzelmann-Schwarz V., Wiese M., Lardinois D., Umana P., Klein C., Laubli H., Kashyap A.S., Zippelius A. Fibroblast activation protein-targeted-4-1BB ligand agonist amplifies effector functions of intratumoral T cells in human cancer. J. Immunother. cancer. 2020 doi: 10.1136/jitc-2019-000238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, M., Hong, Y., Feng, Q., Pan, X., Song, S., Cui, J., Lei, J., Fang, H., Yang, J., 2018. Recombinant Adenovirus KGHV500 and CIK Cells Codeliver Anti-p21-Ras scFv for the Treatment of Gastric Cancer with Wild-Type Ras Overexpression. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2018.10.003. [DOI] [PMC free article] [PubMed]
- Yamada S., Ritchim P., Charkrabandhu T., Jongraksat W. Combination 5-fluoruracil/cisplatinum versus 5-fluoruracil/leucovorin adjuvant chemotherapy efficacy for R0 gastric resection in locally invasive gastric cancer. J Med Assoc Thai. 2012 [PubMed] [Google Scholar]
- Yao, Q., Qi, X., Cheng, W., Xie, S.H., 2019. A comprehensive assessment of the racial and ethnic disparities in the incidence of gastric cancer in the United States, 1992–2014. Cancer Res. Treat. https://doi.org/10.4143/crt.2018.146. [DOI] [PMC free article] [PubMed]
- Yi J.H., Kang J.H., Hwang I.G., Ahn H.K., Baek H.J., Lee S. Il, Lim D.H., Won Y.W., Ji J.H., Kim H.S., Rha S.Y., Oh S.Y., Lee K.E., Lim T., Maeng C.H., Kim M.J., Kim S.T., Lee J., Park J.O., Park Y.S., Lim H.Y., Kang W.K., Park S.H. A retrospective analysis for patients with HER2-positive gastric cancer who were treated with trastuzumab-based chemotherapy: In the perspectives of ethnicity and histology. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W., Park K.B., Chung H.Y., Kwon O.K., Lee S.S. Chronological changes of quality of life in long-term survivors after gastrectomy for gastric cancer. Cancer Res. Treat. 2016 doi: 10.4143/crt.2015.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue, B., Song, C., Yang, L., Cui, R., Cheng, X., Zhang, Z., Zhao, G., 2019. METTL3-mediated N6-methyladenosine modification is critical for epithelial-mesenchymal transition and metastasis of gastric cancer. Mol. Cancer. https://doi.org/10.1186/s12943-019-1065-4. [DOI] [PMC free article] [PubMed]
- Zhang, H., Deng, T., Liu, R., Ning, T., Yang, H., Liu, D., Zhang, Q., Lin, D., Ge, S., Bai, M., Wang, X., Zhang, L., Li, H., Yang, Y., Ji, Z., Wang, H., Ying, G., Ba, Y., 2020. CAF secreted miR-522 suppresses ferroptosis and promotes acquired chemo-resistance in gastric cancer. Mol. Cancer. https://doi.org/10.1186/s12943-020-01168-8. [DOI] [PMC free article] [PubMed]
- Zhou, Z., Zhang, H., Deng, T., Ning, T., Liu, R., Liu, D., Bai, M., Ying, G., Ba, Y., 2019. Exosomes Carrying MicroRNA-155 Target Forkhead Box O3 of Endothelial Cells and Promote Angiogenesis in Gastric Cancer. Mol. Ther. - Oncolytics. https://doi.org/10.1016/j.omto.2019.10.006. [DOI] [PMC free article] [PubMed] [Retracted]