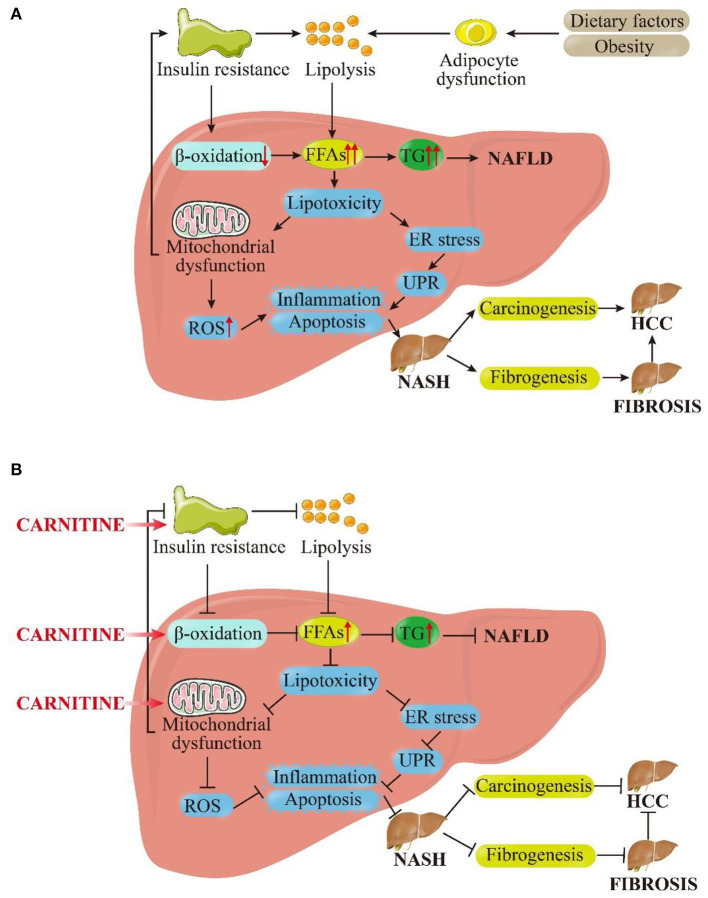
Figure 3.
Pictorial representation of the (A) pathophysiological mechanism of NAFLD based on the “multiple hit” hypothesis and (B) inhibitory effects of carnitine on NAFLD and its progression. As shown in (A), dietary and environmental factors, together with obesity, lead to the proliferation and dysfunction of adipocytes. Insulin resistance acts on adipose tissue and worsens adipocyte dysfunction, which in turn worsens lipolysis. In the liver, insulin resistance inhibits β-oxidation. Under the dual attack of increased lipolysis and weakened β-oxidation, the influx FFAs to hepatocytes is increased greatly, leading to the synthesis and accumulation of TG and enhanced liver lipotoxicity. Excessive TG eventually induces NAFLD. Increased lipotoxicity leads to mitochondrial dysfunction and oxidative stress on endoplasmic reticulum (ER) by the activation of ROS, which leads to liver inflammation and fibrosis. At the same time, mitochondrial dysfunction promotes insulin resistance, exacerbating above process in a vicious circle. These pathological processes can cause the liver to persist in the stable stage of disease (NAFLD) or develop in to NASH. In the late stage of disease progression, NASH can progress to fibrosis or even HCC under the stimulation of certain factors. As shown in (B), carnitine supplementation reduces insulin resistance, promotes β-oxidation and improves mitochondrial function. Subsequently, the cellular concentration of FFAs and TG in hepatocytes get reduced and lipotoxicity is alleviated. The level of ROS is restrained to a certain extent, and inflammation and apoptosis were also improved. These above interlocking effects could alleviate NAFLD and NASH, and they may even have positive therapeutic effects on liver fibrosis and HCC. FFAs, free fatty acids; TG, triglycerides; ER, endoplasmic reticulum; UPR, unfolded protein response; ROS, reactive oxygen species; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma.