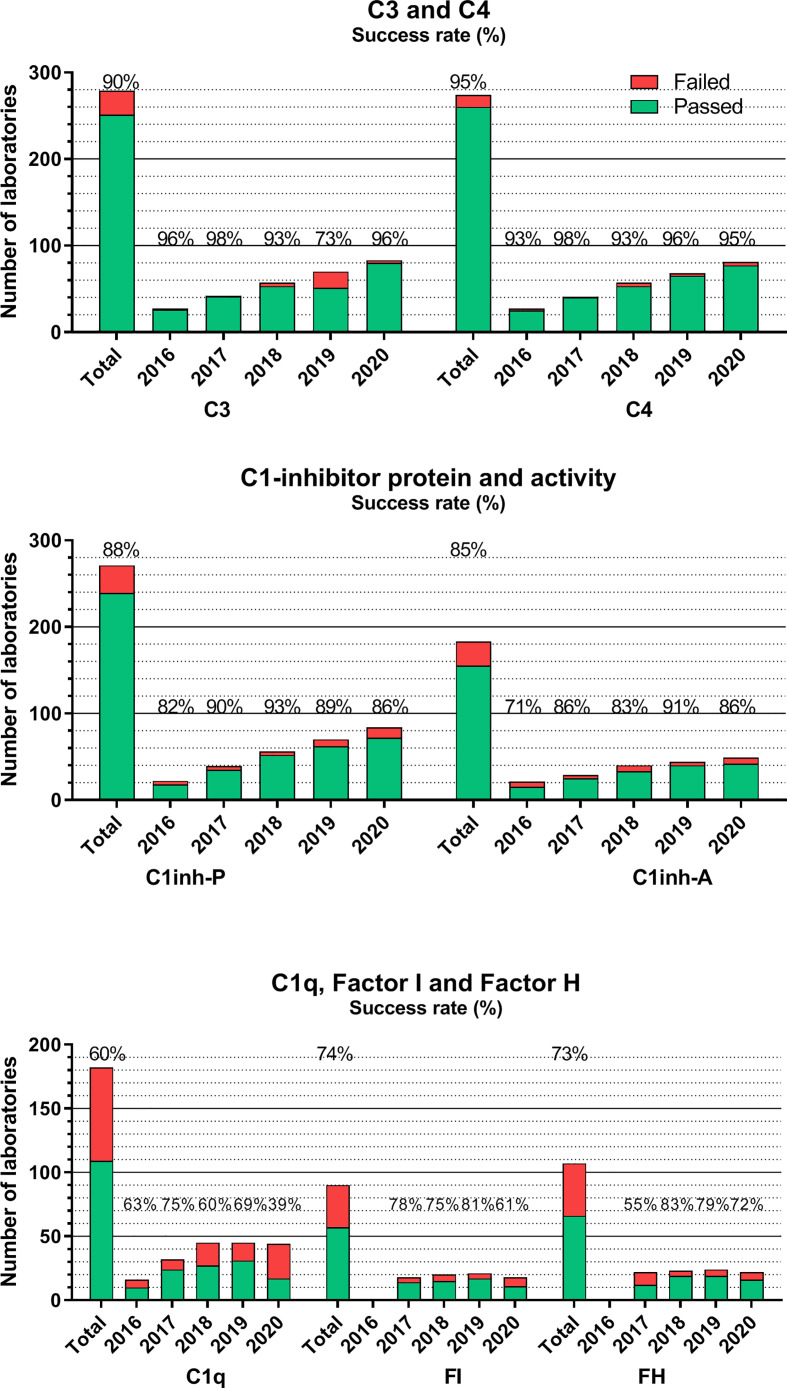
Figure 3.
Number of laboratories that “failed” or “passed” in the given year in the EQA program for C3, C4, C1-INH protein, and activity, C1q, factors H and I. Success rate was calculated as frequency of laboratories with ‘passed’ results among all the participants. “Total” indicates the average success rate for the whole group in the past 5 years (2016–2020). Note, that laboratories using commercial nephelometry or radial immunodiffusion (RID) assays have consistently better success rates than laboratories using in-house ELISA or homemade RID. The lack of uniform calibration and a frequent use of ill-defined “units”/ml, both excluded the possibility to evaluate such results in the EQA program (the size of the homogenous method/dimension groups is too low). This is a factor in the increasing proportion of laboratories without certificate.