Scheme 1.
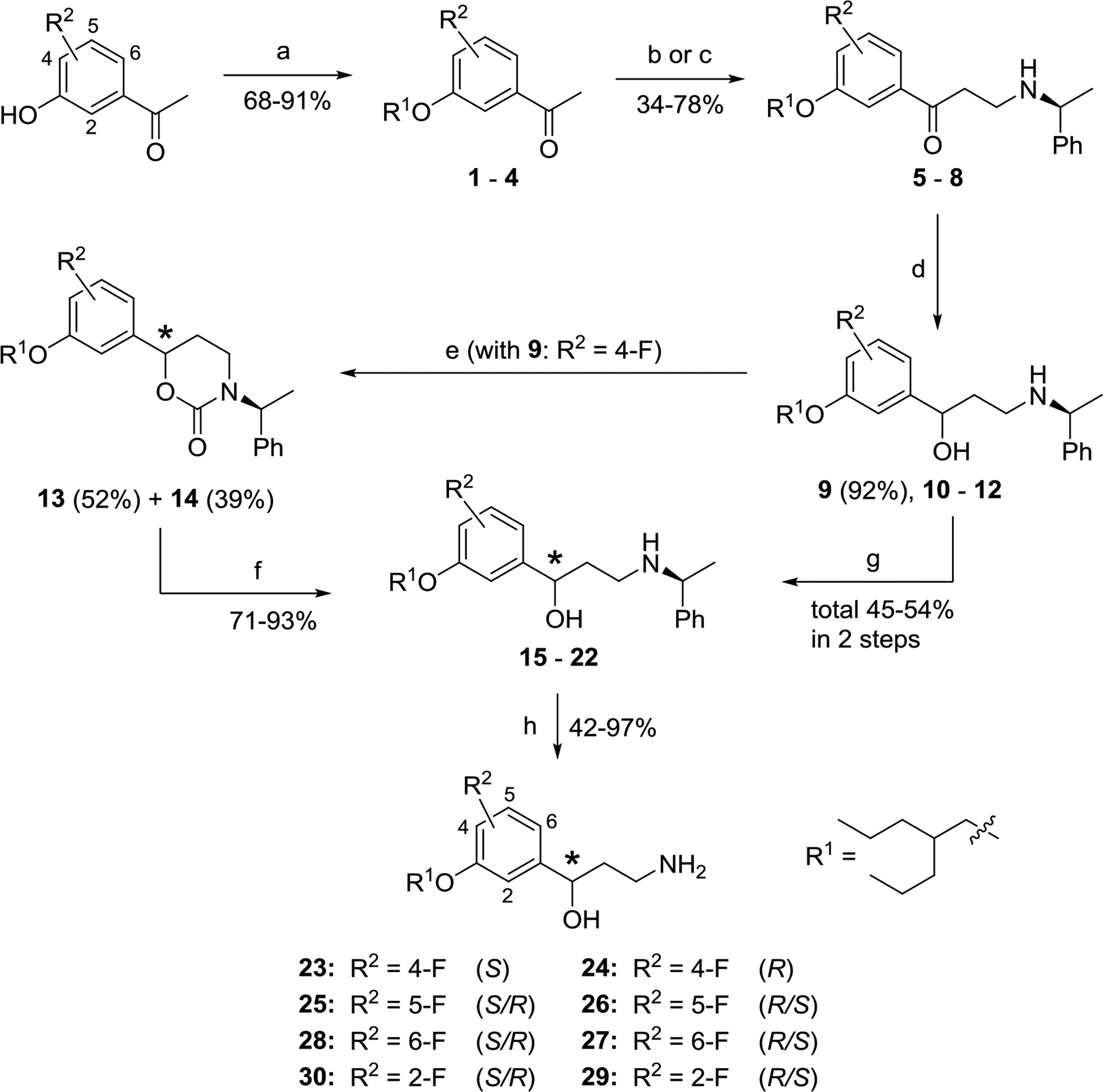
Synthesis and asymmetric resolution of (2-propylpentyl)oxyfluorophenyl analogs of emixustat 23–30. a
aReagents and conditions: (a) 2-Propylpentylmesylate, K2CO3, DMF, 85 °C, 2 – 4 h. (b) Paraform, (S)-(‒)-methylbenzylamine, conc. HCl (cat.), 1,4-dioxane, MW, 100 – 130 °C, 5 min. (c) 1,3,5-Trioxane, (S)-(‒)-methylbenzylamine, conc. HCl (cat.), 1,4-dioxane, sealed tube, 110 °C, overnight. (d) NaBH4, MeOH, r.t., 1 h. (e) (i) CDI, THF, reflux, overnight; (ii) separation of diastereomers by FC on SiO2. (f) KOH, EtOH, reflux, overnight. (g) Separation of diastereomeric mixtures 10 – 12 by chiral HPLC. (h) Ammonium formate, Pd/C (cat.), MeOH, reflux, 30 min. – overnight.
