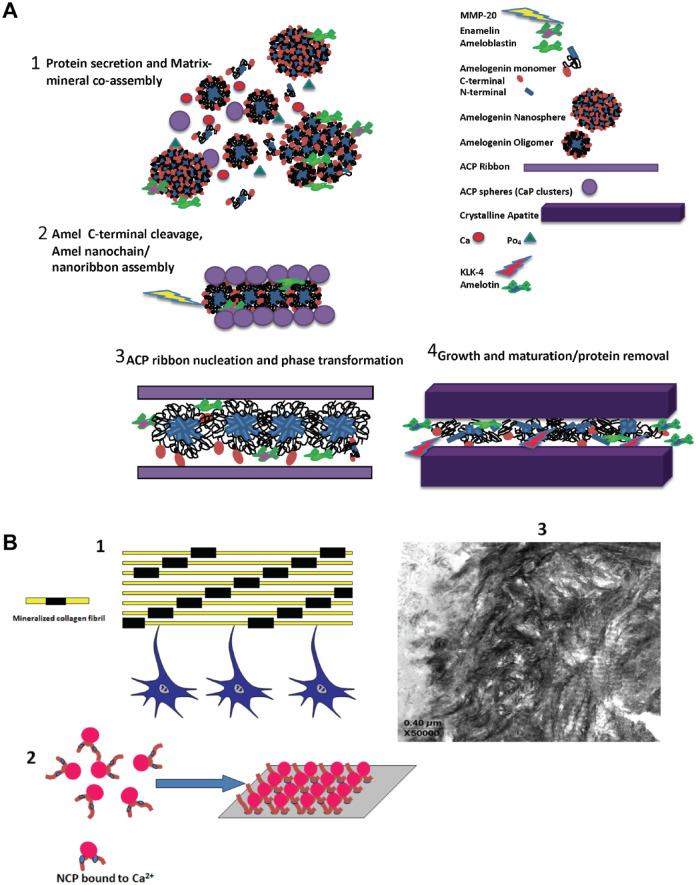
Figure 1.
Protein–mineral assembly in enamel and dentin. (A) A proposed conceptual model for protein-mediated enamel biomineralization in a continuously forming amelogenin-based matrix. (1) Amelogenin molecules are secreted in monomeric or oligomeric forms (dimers, trimers, hexamers) and spontaneously assemble into transient nanospheres (Moradian-Oldak 2012; Shaw et al. 2020). Amelogenin-mineral coassembly stabilizes amorphous calcium phosphate (ACP) particles (Wiedemann-Bidlack et al. 2011). Nanospheres may contain enamelin and ameloblastin molecules (Fan et al. 2009; Mazumder et al. 2014; Bapat et al. 2020). (2) Amelogenin C-terminus is cleaved by MMP-20 (Lu et al. 2008). Oligomers spontaneously assemble into elongated nanochains, promoting oriented nucleation of calcium phosphate clusters (Moradian-Oldak 2012). A model for nanoribbon-like structures has been also proposed and might be formed at this stage (Engelberth et al. 2018). (3) Calcium phosphate clusters are fused to form elongated ACP ribbons and later transform to crystalline apatite. ACP–apatite transformation is facilitated following the C-terminal cleavage of amelogenin. Amelotin, which is expressed during maturation stage, facilitates crystal growth. (4) With the cleavage of the amelogenin N-terminus by MMP-20, the disassembly of the nanospheres is promoted, and further degradation of amelogenin and other matrix proteins takes place in the maturation stage by the serine proteinase (KLK-4), allowing further growth of apatite mineral in thickness. (B) Dentin and bone tissues are formed by matrix-mediated mineralization: (1) Cartoon showing dentin and bone formation is a cellular event. Components secreted by osteoblasts/odontoblasts are responsible for calcified tissue formation. Yellow bars represent collagen fibrils; black bars represent calcium phosphate (CaP) mineral in the gap region. (2) Cartoon showing that noncollagenous proteins (NCPs) have the ability to bind Ca2+. These nanoclusters presumably localize on self-assembled templates such as collagen and provide the structural surface for stereospecific mineral nucleation. (3) Representative unstained TEM image of an unerupted bovine molar showing mineralized collagen fibrils.