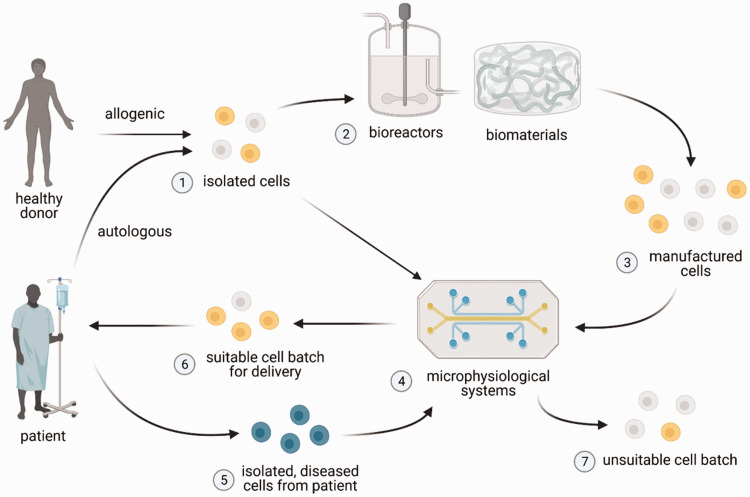
Figure 1.
Potential utility of bioreactors, biomaterials, and microphysiological systems for cell manufacturing. Cells (1) are first isolated and purified from autologous (patient-derived) or allogenic (healthy donor) source, after which they can be grown and expanded into therapeutic cell numbers in bioreactors and/or biomaterials (2) that provide distinct microenvironmental cues. The expanded cells (3), however, may be heterogenous in their quality attributes. Microphysiological systems (4), which may incorporate patient-specific diseased cells (5) (i.e. cancer cells), can be used to characterize isolated cells (1) (in cases where expansion is not necessary) or manufactured cells (3), in order to identify batches of cell product that have suitable (6) or unsuitable (7) quality attributes for use in patients. Figure created with BioRender.com. (A color version of this figure is available in the online journal.)