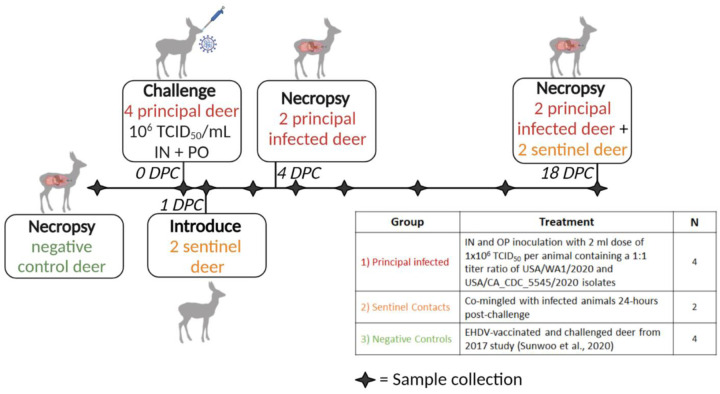
Figure 1. Experimental design.
Ten female white-tailed deer were split into three groups as follows: i) four principal infected deer, ii) two sentinel contact deer, and iii) four non-inoculated control deer. Group 1 was inoculated simultaneously via intra-nasal and oral routes with a 2 ml dose of 1×106 TCID50 per animal containing an approximate 1:1 titer ratio of the lineage A WA1 strain and an alpha VOC B.1.1.7 strain of SARS-CoV-2. Group 2 deer (n=2) were used as sentinel contact animals and were not challenged directly. These sentinel deer were placed up air current of the room’s directional airflow and separated from the principal infected group by an 8-foot tall, solid partition wall on the day of challenge, provided separate food and water, and re-introduced to principal infected (group 1) 24-hours post infection. Nasal, oral, and rectal swabs were collected on days 0, 1, 3, 5, 7, 10, 14, and 18 post-challenge. Whole blood and serum were collected on 0, 3, 7, 10, 14, and 18 DPC. Two principal infected deer (group 1) were euthanized for postmortem examination on 4 days-post-challenge (DPC) to evaluate the acute phase of infection. The four remaining deer, consisting of two sentinels and two principal infected, were maintained for the duration of the 18-day study to evaluate contact transmission and the convalescent stage of infection. The control deer were part of a separate study (36).