Figure 7 |. Engineering non-natural carbene transferases for biocatalytic C–C bond formation.
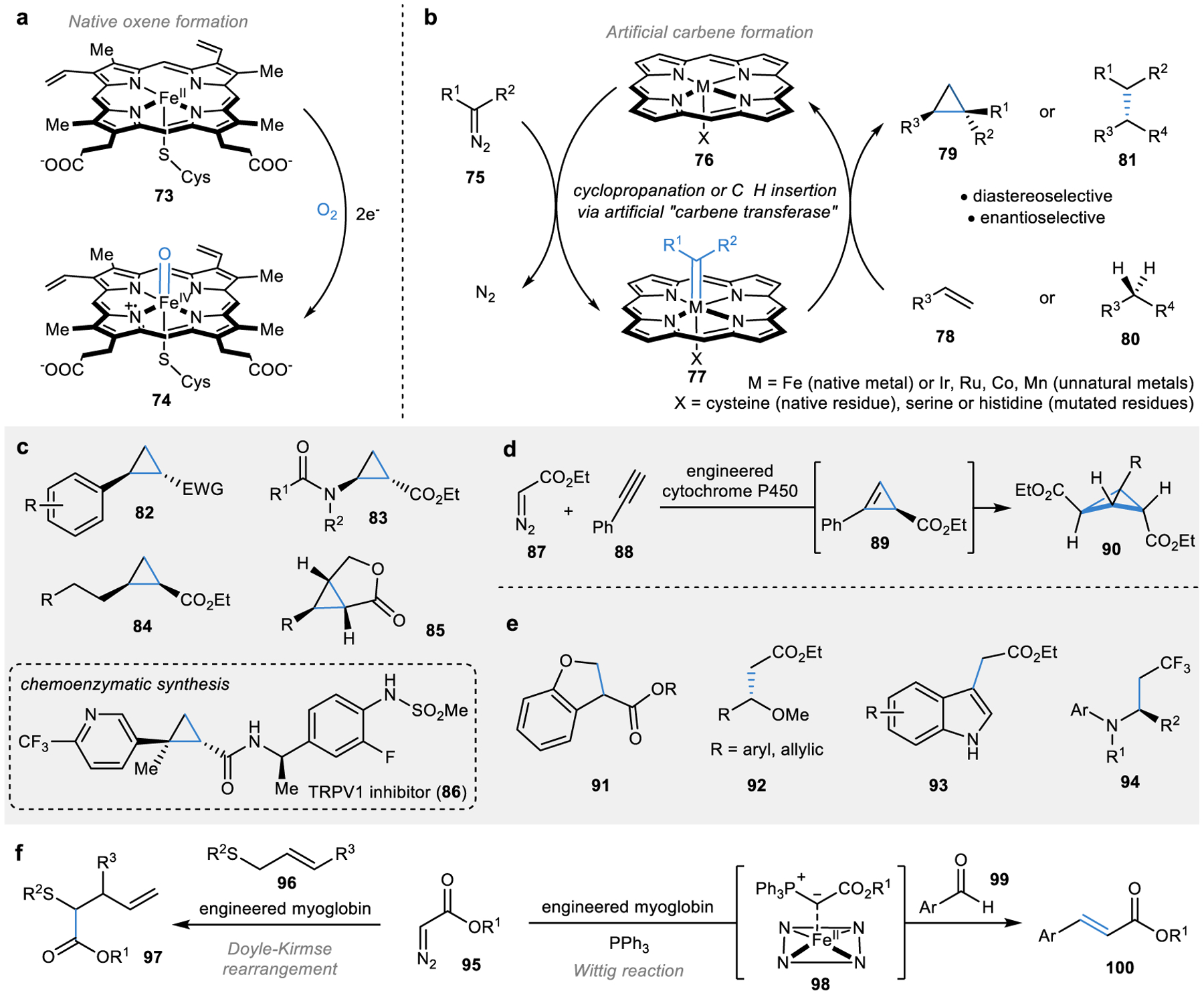
a | Natural iron-oxene formation in the haem cofactor of haemoproteins. b | Engineering artificial carbene-transferases through iron-carbene formation.151,168–170 c | Scope of cyclopropanation reactions catalysed by carbene transferases158–160,162–164 and representative use in chemoenzymatic synthesis.166 d | Carbene transfer into alkyne forms highly strained cyclopropene and bicyclobutane products.165 e | Carbene insertion into C–H bonds forms alkylated products.168,169,171–174 f | Carbene transferases have catalysed C–C bond formation through rearrangement reactions and Wittig reactions.175–177
