Figure 3.
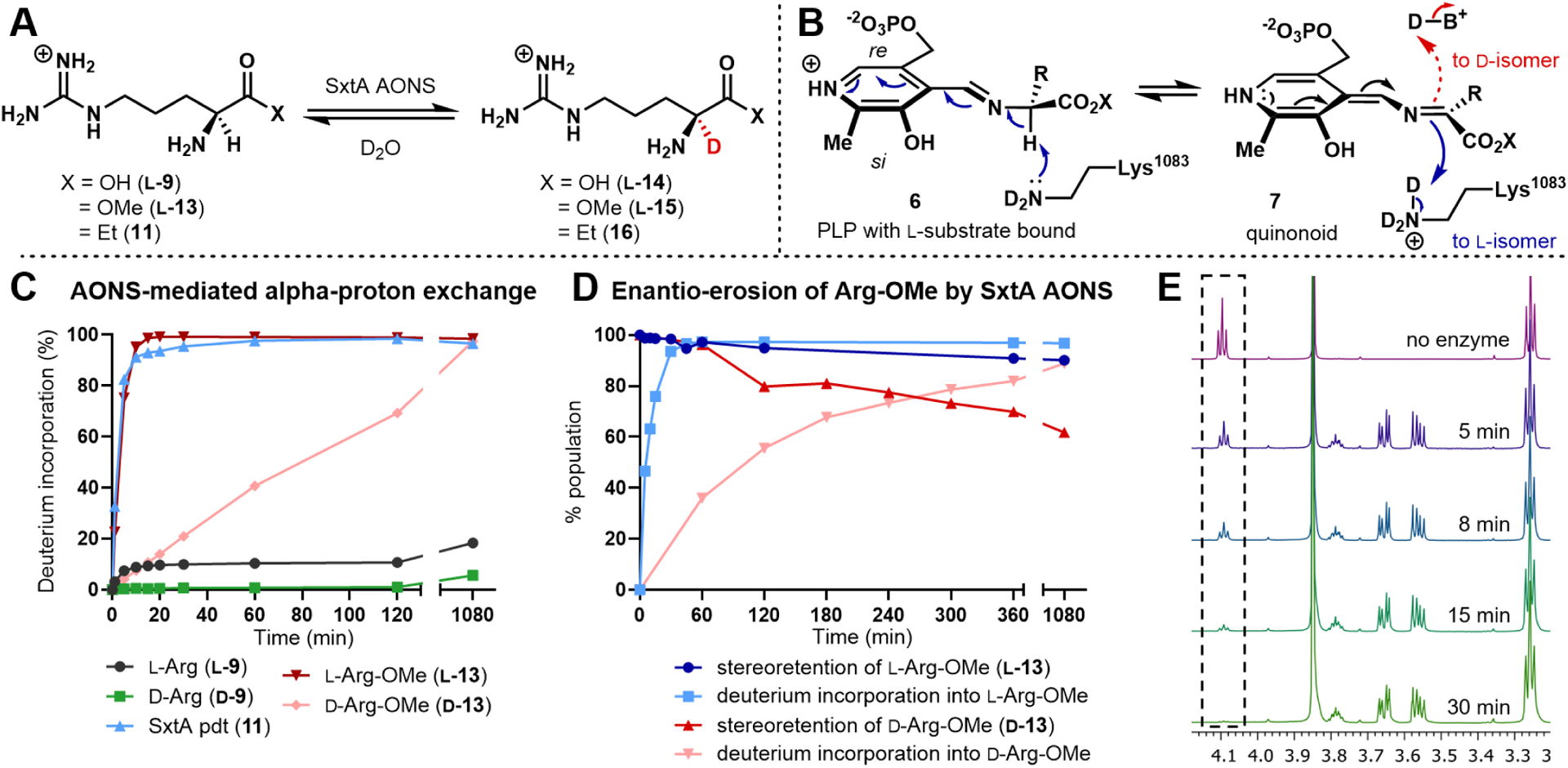
Deuteration of arginine-related substrates. (A) α-deuterated compound formation. (B) Potential mechanisms of proton/deuterium transfers in SxtA AONS. (C) Timecourse of α-deuteration by mass spectrometry. (D) Comparison of deuterium incorporation and enantiomeric composition when starting with l-Arg-OMe (blue) or d-Arg-OMe (red). (E) 1H NMR of l-Arg-OMe (l-13) incubated with AONS over 30 min, confirming exchange of the α-proton to deuterium (see SI for conditions). Peaks present between 3.7−3.5 ppm are attributed to glycerol from the enzyme storage buffer.
