Abstract
The utilization of medicinal plants and their derivatives in treating illnesses is more appropriately recognized as herbal remedy than traditional medicine. For centuries, medicinal herbs have been used for the treatment of diseases in many countries. Malva sylvestris L. is a kind of mallow derived from Malvaceae species and is recognized as common mallow. This amazing plant has antimicrobial, hepatoprotective, anti-inflammatory, and antioxidant properties and is considered as one of the most promising herbal medicinal species. This plant's traditional use in treating many diseases and preparing pharmaceutical compounds can show us how to know in depth the plant origin of drugs used to produce antibiotics and other therapeutic agents.
1. Introduction
Malva sylvestris L. (M. sylvestris) is one of the medicinal plants commonly recognized as common mallow in Europe, Iran, Pakistan, and India. M. sylvestris is a biennial-perennial herbaceous plant commonly found in North Africa, Europe, and Southwest Asia [1, 2]. The plant generally grows in moist areas, for instance, near marshes, ditches, oceans, riverbanks, and meadows [3]. Due to the softening properties of this plant, the Romans and ancient Greeks used it as a softener [4, 5]. Traditionally, these medicinal plants have been used to treat several infections and diseases, such as cold, burn, cough, tonsillitis, bronchitis, digestive problems, eczema, and cut wounds under different weather conditions [6]. As a natural product, M. sylvestris leaves and flowers showed various therapeutic effects. Figure 1 shows some of the medicinal applications of this plant.
Figure 1.
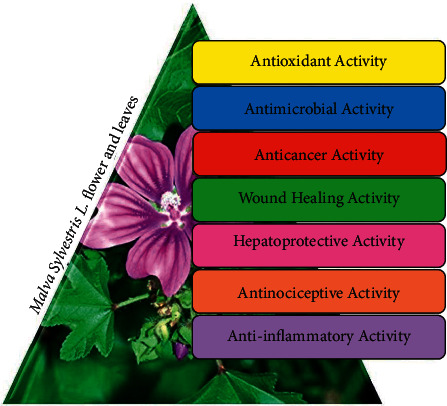
Flower of M. sylvestris and different biological activities [7].
Fluid extracts of M. sylvestris leaves and flowers are used to treat inflammatory diseases of mucous membranes, cystitis, and diarrhea [8]. This plant derives its restoration competencies from the mucilage and flavonoids located in the vegetation and leaves. Young leaves, shoots, flowers, and fruits are consumed in salads, soups, or boiled root vegetables. Flowering flora in the mallow family (Malvaceae) or hibiscus generally include the Malva and Hibiscus species. Hibiscus species comprise the swamp-rose mallow (Hibiscus moscheutos); another type of rose mallow (Hibiscus militaris), a shrub that grows to a peak of 2 m; and superb rose mallow (Hibiscus grandiflorus), with ample white to purplish flowers. Herbal medicine is one of the oldest treatment practices followed by humans. In the last 30 years, medicine specialists focus on the use of medicinal herbs in preventing and treating diseases. Among the numerous species used in traditional medicine, Malvaceae family is more prominent due to its diverse applications, and its consumption can be traced back to 3000 years ago.
The marshmallow (Althea officinalis), generally found in swamplands or marshes near the sea, is indigenous to North America and local to Europe and North Africa. Recently, its root has been used to make sweets. Malva plant in India, with a maximum height of up to 40 cm, is prescribed for the treatment of cough and cold due to respiratory problems involved and for the treatment of gastrointestinal problems [9]. This drug is used in Brazil to treat bronchitis, wounds, colitis, and hemorrhoids [10]. The chemicals in the leaf of Malva, which has many vitamins, allow for faster recovery by secreting certain analgesics to reduce pain and discomfort [11].
Medicinal plants have been frequently used to treat a variety of human diseases. Over the last century, the use of vegetation in medication, hematology, oncology, and immunology has affected the identity of natural composites: codeine, taxol, vinblastine, morphine, and cocaine, among others. The results of several studies have shown that Malva extract contains different compounds, including phenolic derivatives, flavonoids, terpenoids, catalase enzymes, sulfite oxidase, fatty acids, and certain strolls (specifically essential fatty acids such as omega-3 and omega-6), beta carotene, and vitamins C and E, which have anti-inflammatory and antioxidant properties [12–15]. Therefore, it can protect the kidney against injuries due to renal toxicity resulting from the cisplatin and vanadium system [16]. Extensive research shows that this plant, with different chemical compounds, can minimize liver damage caused by carbon tetrachloride. M. sylvestris has antimicrobial, antinociceptive, hepatoprotective, wound-healing, anticancer, anti-inflammatory, and potent antioxidant properties (Figure 2). Also, this plant contains many valuable compounds such as strong antioxidants and carbohydrates and unsaturated fatty acids. Tannins, flavonoids, phenolic compounds, and ascorbic acid found in the Malva plant are used to treat most cancers and for wound-healing [2, 7, 9, 17].
Figure 2.
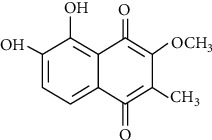
Chemical framework of malvone A, a phytoalexin found in M. sylvestris [7].
1.1. Phytochemistry
The prevalence of using plant antioxidants, considering their use in various research and applied aspects of antioxidants, especially the valuable compounds underlying phenolic induction with its groups with free radical absorption, plays an essential role in spreading its use as an oxidation preventive agent [18]. In the study conducted by Nawwar et al. [19], the phenol carboxylic and free organic acids were methylated. By using the following formula, the contents of components were calculated:
| (1) |
where K1=A1/A2 (A1 and A2 are the peak areas of the test and standard compounds, respectively) and K2 is the mass of the internal standard (μg) added to the sample. The component combination of organic acids is shown in Table 1.
Table 1.
Quantitative contents of organic acids in leaves of M. sylvestris [20].
| Acid | Retention time (min) | Content (mg/kg) |
|---|---|---|
| Oxalic | 8.88 | 4170.7 |
| Malonic | 11.13 | 1284.4 |
| Fumaric | 11.97 | 6924.8 |
| Succinic | 12.95 | 644.9 |
| Benzoic | 13.96 | 60.1 |
| Glutaric | 15.51 | 37.7 |
| Phenylacetic | 16.62 | 103.6 |
| Salicylic | 16.93 | 219.0 |
| Malic | 21.32 | 3510.0 |
| Citric | 28.46 | 13133.2 |
| Vanillic | 31.33 | 84.3 |
| Ferulic | 38.99 | 397.7 |
| p-Coumaric | 39.73 | 65.9 |
A total of 13 organic acids extracted from the leaves of M. sylvestris are known, including malonate (1284.4 mg/kg), malate (3510.0 mg/kg), oxalate (4170.7 mg/kg), fumarate (6924.8 mg/kg), and citrate (13133.2 mg/kg). These compounds contribute to developing the immunostimulant and antioxidant properties for M. sylvestris and their preparations based on these natural compounds [5, 20]. It is proven that these flavonoids structures, along with other phenolic compounds, are present in higher amounts in the M. sylvestris flowers and have more effective antioxidant properties, as given in Tables 1 and 2. The antioxidant property was found to be more profound in flower extracts of M. sylvestris based on the results of the (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical method (DPPH assay) and ferric reducing antioxidant power assay (FRAP assay). The researchers further discovered more flavonoids and phenolic contents and antioxidants in leaves than in leafy flower stems and flowers when 95% ethanol was removed [21].
Table 2.
In vitro antioxidant activity of M. sylvestris flowers and leaves, complete flavenoid content, and total phenolic content (TPC) [21].
| Sample | TPC (mg GAE/g FW) | Total flavonoids (mg QE/g FW) | FRAP | DPPH |
|---|---|---|---|---|
| mM TE/g FW | ||||
| Mallow leaves | 1.42 ± 0.14 | 0.76 ± 0.19 | 4.04 ± 0.85 | 3.88 ± 0.51 |
| Mallow flowers | 6.32 ± 0.13 | 1.45 ± 0.21 | 6.01 ± 0.54 | 5.98 ± 0.43 |
A major phytoalexin found in M. sylvestris was 2-methyl-3-methoxy-5,6-dihydroxy-1,4-naphthoquinone, known as malvone (Figure 2). Figure 3 shows some flavonoids that have a significant therapeutic effect.
Figure 3.
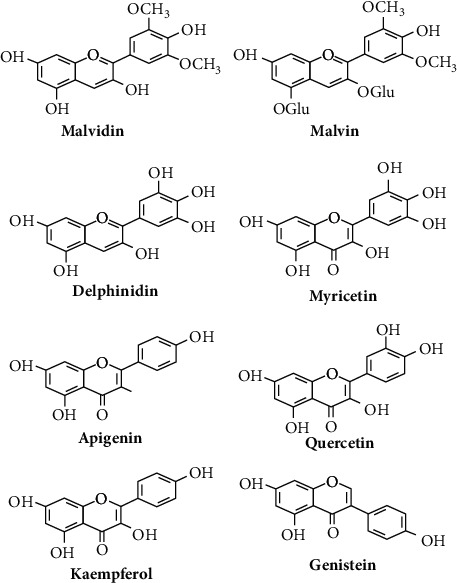
Some flavonoids of M. sylvestris [7].
1.2. Carbohydrate Content in M. sylvestris
Research has shown that most carbohydrates in plant materials derived from polysaccharides demonstrate an unknown mechanism during antioxidant activity. In animal experiments, these polysaccharides, especially pectins, are mainly found in plant tissues, show antioxidant and antidiabetic properties, and even adjust blood insulin, as given in Table 3.
Table 3.
In vitro antioxidant activity, total flavenoid content, and total phenolic content of M. sylvestris leaves and flowers [21].
| Sample | Fructose | Glucose | Sucrose | Reducing sugars | Total soluble carbohydrates |
|---|---|---|---|---|---|
| Mallow leaves | 0.88 | 0.61 | 0.46 | 2.1 | 42.9 |
| Mallow flowers | 2.03 | 0.93 | 0.21 | 5.5 | 47.0 |
The leaves are the richest in flavonoids, and this explains their therapeutic properties in traditional medicine.
1.3. Mucilages
The mucilages contain trehalose, galactose, sucrose, glucose, fructose, mannose, rhamnose, galacturonic, and glucuronic acid, but 2″-O-a-(4-O-methyl-a-d-glucuronosyl)-xylotriose, raffinose, fucose, xylose, arabinose, and uronic acid have also been found in M. sylvestris. It is considered an essential antimicrobial agent due to its resistance to the pathogen Verticillium dahliae [22].
1.4. Pigments
Qualitative analysis of acetone extracts from M. sylvestris has been done using chromatography. These assessments approve the presence of xanthophylls, chlorophyll B, and chlorophyll A [23].
1.5. Fatty Acids/Sterols
In M. sylvestris leaves, the presence of the stigmasterol, g-sitosterol, and the steroid campesterol has been reported [22]. The plant growth status affects the qualitative and quantitative constituents of these materials. Lipids exist separately in the flowering stems, immature fruits, flowers, and leaves [2]. These include tricosanoic acid, heneicosanoic acid (C20:3n3 + C21:0), lignoceric acid, 14-eicosadienoic acid, cis-11, behenic acid, arachidic acid, eicosenoic acid, a-linolenic acid, linoleic acid, heptadecanoic acid, palmitoleic acid, pentadecanoic acid, oleic acid, stearic acid, myristic acid, palmitic acid, myristoleic acid, lauric acid, capric acid, caprylic acid, and caproic acid. Extracts from leaf upon rapid cure with methanol and acetyl chloride contain 0.47% lipids and linolenic acid (42.21%). Because of the availability of indispensable fatty acids such as omega-3 and omega-6, M. sylvestris plays a pivotal role as a nutraceutical food. The consumption of omega-3 fatty acid compounds can prevent many diseases, such as coronary artery disease, diabetes, and cancer.
1.6. Chemical Elements
Assessment of the leaves of M. sylvestris has shown the presence of essential and nonessential metallic elements, halogens, and nonmetals. Analysis was performed using plasma optical emission spectrometry (ICP-OES), and the presence of Zr, Zn, U, Tl, Sr, Pb, Ni, Na, Mn, Mg, Sn, La, K, Si, Fe, Cu, Cr, Co, Ca, Bi, Ba, B, and Al was also shown [24]. M. sylvestris has exhibited a considerable ability to accumulate substantial metals (Zn, Pb, Ni, Cu, and Cd) from soils rich in these materials. Thus, it is crucial to address this issue in affected populations living in hazardous zones [25].
1.7. Vitamins
One of the natural properties of M. sylvestris is the human cell supplementation using ascorbic acids (vitamin C) and tocopherols (vitamin E). Vitamin E is considered a remarkable cancer prevention agent of the tocopherols in the human body [2, 26].
1.8. Enzymes
In the oxidative degradation of sulfur-containing amino acids, sulfite oxidase as an enzyme plays an integral role in ending the reaction (Figure 4). The absence of this enzyme might lead to death. Sulfite oxidase has additionally been discovered in the leaves of M. sylvestris and has been found in numerous bacteria and animal species [26–29]. Various phenolic derivatives have been found in extracts from different parts of M. sylvestris [26, 27].
Figure 4.
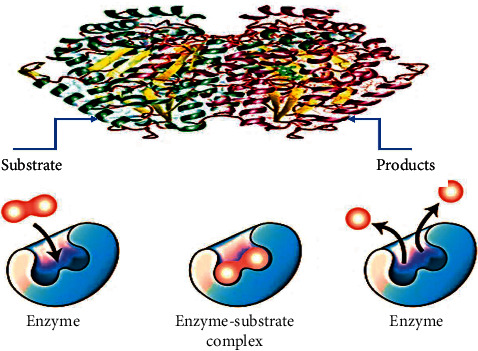
Mechanism of enzyme activity.
1.9. Pharmacological Activity
M. sylvestris has been reported for use in the therapy of oral diseases. Anti-inflammatory and antimicrobial effects on the antimicrobial outcomes of ethanolic extracts from M. sylvestris stems were investigated in contrast to methicillin-resistant Staphylococcus aureus through biofilm adherence/formation tests and planktonic growth [30].
The biofilm foundation method showed that ethanolic stem extracts had medium activity in planktonic growth tests against S. aureus with bounded bacteriostatic effects [30–32]. Ethanolic extracts obtained from the inflorescences and leaves of M. sylvestris have a significant impact on Helicobacter pylori. This bacterial strain plays an essential role in treating peptic ulcers and gastric cancers [33].
Hence, in the agar, diameter inhibition zones, crude methanolic extracts did not significantly inhibit strains of Saccharomyces cerevisiae, Bordetella bronchiseptica, Candida albicans, Serratia marcescens, B. pumilus, B. cereus, B. subtilis, M. luteus, S. epidermidis, K. pneumonia, S. aureus, E. coli, P. fluorescens, and P. aeruginosa [34–36]. The useability of M. sylvestris in mice considering the aqueous extract as an anti-inflammatory agent has been investigated. Research has shown that this type of extract might significantly reduce inflammation. The hydroalcoholic extract received from M. sylvestris leaves exhibited an anti-inflammation effect on croton oil-induced swelling in the ears of mice. The extract outcome has been proven by these facts [37].
The pharmacological activities of M. sylvestris are summarized in Table 4. Claimed patents are listed in Table 5. Other details related to the medicinal use of M. sylvestris are given in Table 6.
Table 4.
M. sylvestris pharmacological activities.
| Activity | Models | Extract/pharmaceutical preparations | Findings |
|---|---|---|---|
| Biochemical profile | Extract intake in rats by drinking water | Aqueous extract from aerial parts | Bodyweight dosages (400 and 800 mg/kg) resulted in a significant rise in serum triglycerides, while other lipids, liver enzyme parameters, and glycaemic (alanine and aspartate transaminases, alkaline phosphatase, lactate dehydrogenase) were unaffected [35] |
|
| |||
| Bioadhesive mucous membranes | Ex vivo system (mucous membranes prepared buccal region tissue from killed pigs) | Aqueous extracts (flowers) | Less bioadhesion for epithelial tissue. Not feasible to correlate rehydration effects in this study, anti-irritative and anti-inflammatory [38] |
|
| |||
| Antiaging | Quantitative reverse transcriptase-PCR (polymerase chain reaction) and DNA macro array | Extract from seed | The rise in antioxidant gene expression [39] |
|
| |||
| Antimicrobial | Sequential dilution of plant extracts mixed with 1 ml of DPPH | Methanolic extracts (seeds), dichloromethane, and n-hexane | Antioxidant properties by thin-layer chromatography (TLC) qualitative plates test. For the DPPH test, no low activities for methanolic and n-hexane extracts were observed, and there was no activity for dichloromethane extract [40] |
|
| |||
| Anticancer | MTT test | Hydroalcoholic leaves extract | Notable proliferative reduction of A375 and B16 cancer cell lines [41] |
|
| |||
| Acetylcholinesterase (AChE) | The activity of enzymes evaluated at visible wavelengths | Ethanolic extract, essential oil fraction, decoction, and from aerial portions | No inhibitory observed through the use of the ethanolic extract, and 25% inhibited using 5 mg/ml of plant decoction; 28% of AChE inhibition by 0.1 mg/ml of essential oil [18] |
Table 5.
Pharmacological activities of M. sylvestris proclaimed in patents.
Table 6.
Other related medicinal uses of M. sylvestris.
| General use | Parts used | Preparation | Specific use |
|---|---|---|---|
| Vaginal disorders | Flowers and leaves | Decoction | Vaginal itching [43] |
| Pain | Root and leaves | The vapor of decoction (M. sylvestris association) | Lumbar ache [44] |
| Urological disorders | Fruit | Infusion | Irritation of urinary organs, protector of bladder mucous [45] |
|
| |||
| Respiratory complaints | Fruit | Infusion | Cough [46] |
| Aerial parts | Decoction | Respiratory diseases, cough, sore throat, bronchitis [47, 48] | |
| Leaves/flowers | Leaves/flowers | Pectoral asthma, spasmolytic, expectorant, cough, and emollient [49, 50] | |
| Whole plant | Infusion | Chronic bladder ulcer, bladder pains [46, 51, 52] | |
|
| |||
| Inflammation | Leaves, flowers, and whole plant | A crushing plant | Rheumatism, the local application against arthritis [53] |
|
| |||
| Haemorrhoidal | Leaves | Vapour, infusion | Antihaemorrhoidal [54, 55] |
|
| |||
| Dermatological ailment | Flowers and leaves | Infusion, decoction | Astringent, acne [49, 56] |
| Decoction | Emollient | Roots [57] | |
|
| |||
| Menstrual pains | Roots | Decoction | Menstrual pain [58, 59] |
| Flowers | Infusion | Dysmenorrhoea [60] | |
|
| |||
| Gastrointestinal disturbance | Whole plant | Decoction | Laxative effects or depurative, against abdominal pains [61] |
|
| |||
| Other relevant uses | Roots | Decoction | Fever, abortion, weakness, hypertension, and menstrual pain [58, 59, 62] |
| Flowers/leaves | Infusion | Soothing, sedative [49, 63] | |
1.10. Traditional Uses of Malva Species
The traditional-ethnobotanical uses of M. sylvestris are given in Table 7.
Table 7.
Traditional uses of M. sylvestris.
| Country/region | Used part/s | Use/s (reference) |
|---|---|---|
| Iran | Different parts | Cough, expectorant, clear the lung, lubricant, swellings, laxative [64], respiratory diseases of animals, immunomodulation [65] |
| Pakistan | Leaves | Unspecified method: relaxing activity, gastric mucus, anti-inflammatory, indigestion, diuretic, bladder ulcer [66] |
| Algeria | Flower | Infusion: antiseptic for the reproductive system, to treat canker sores, colds, constipation, asthma, otitis, colic, abdominal pain, astringent, antiseptic, softening, insect bites, swelling, boils, abscesses [67] |
| Turkey | Roots | Infusion: abortive [68] |
| Europe | Aerial parts | Constipation, diarrhea, rumination, tympanism, abdominal colic [69] |
| Italy | Leaf, root, flower | Leaves decoction or infusion: bronchitis, weight loss, cold, cough, cystitis, belly pain. Crushed leaves: toothache, whitlow [70] |
| Cyprus | Leaves | Consumed and cooked daily: antidiabetic [71] |
| India | Aerial parts Different parts of the whole plant |
Stimulates the uterus, intestines, ulceration of urinary bladder, cough, enlargement of the spleen, jaundice, sore throat, anti-inflammatory, cooling, mucilaginous [72]. Eaten twice a day to strengthen weak eyesight [73, 74] |
| Brazil | Unspecified | Infusion: tonsillitis wound, rheumatism, uterine inflammation, boil, diuretic, cleanser [75] |
| Slovakia | Aerial parts | Food [76] |
| Syria | Flowers, leaves | Used as a laxative, respiratory infections, cough, mouth wash [77] |
| Portugal | Unspecified | Unspecified method: treatment of infections [61] |
| Spain | Aerial parts | Infusion: Urtica dioica stings and fever, bruises, wounds, laxative [62, 78], cold, kidney malfunction, dysmenorrhoea, gastralgia [60] |
| Morocco | Roots, leaves | Urinary or respiratory disorders, cataplasm or decoction [79] |
| Costa Rica | Whole plant | Unspecified method: ornamental [80] |
| Poland | Fruits | Eaten raw, immature |
| Lebanon | Flowers, leaves | Used to treat arthritis and rheumatism [66] |
Gas chromatography and mass spectroscopy analyses were carried out on compounds found in methanolic leaf extracts of M. sylvestris; results are shown in Table 8 [7].
Table 8.
Major phytochemical compounds detected in the methanolic extract of M. sylvestris.
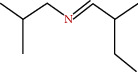
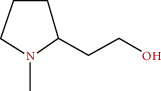
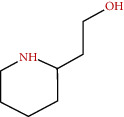
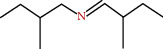
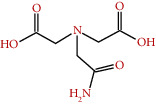
| Serial no. | RT (min) | Molecular weight | Exact mass | Chemical structure | MS fragment ions | Pharmacological actions |
|---|---|---|---|---|---|---|
| 1. | 3.396 | 333 | 333.303165 |
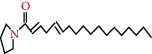
|
55,81,98,1 13,150,220,264,333 |
Unknown |
|
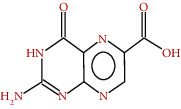
| ||||||
| 2. | 3.218 | 127 | 127.1360993 |
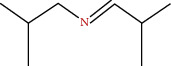
|
57,84,112 | Unknown |
|
| ||||||
| 3. | 3.476 | 78 | 78.013936 |
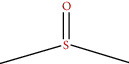
|
63,78 | Anti-inflammatory and antioxidant |
|
| ||||||
| 4. | 3.590 | 127 | 127.1360993 |
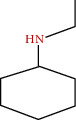
|
55,71,84,9 8,127 |
Anti-inflammatory and antioxidant |
|
| ||||||
| 5. | 3.877 | 141 | 141.15175 |
|
57,69,98,1 13,126 |
Antistereochemistry |
|
| ||||||
| 6. | 4.306 | 129 | 129.115364 |
|
55,84,98,1 29 |
Unknown |
|
| ||||||
| 7. | 4.449 | 129 | 129.115364 |
|
56,84,98,1 28 |
Antimicrobial, antimalarial, antibacterial |
|
| ||||||
| 8. | 4.563 | 155 | 155.167399 |
|
56,70,84,9 8,113,127, 140,154 |
Antimicrobial activity |
|
| ||||||
| 9. | 4.649 | 184 | 184.121178 |
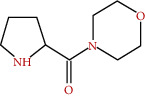
|
56,70,86,1 14,142 |
Antimicrobial activity |
|
| ||||||
| 10. | 5.215 | 191 | 191.043856 |
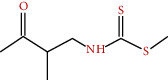
|
57,85,143, 191 |
Antibacterial activity |
|
| ||||||
| 11. | 6.057 | 238 | 238.068868 |
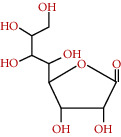
|
61,73,84,1 12,127,142,159,189,220 |
Antibacterial activity |
|
| ||||||
| 12. | 7.041 | 150 | 150.06808 |
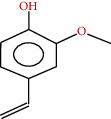
|
51,77,89, 107,135 |
Antioxidant, antimicrobial, and anti-inflammatory |
|
| ||||||
| 13. | 8.025 | 190 | 190.058971 |
|
71,101, 127,146, 172,190 |
Unknown |
|
| ||||||
| 14. | 7.916 | 207 | 207.039239 |
|
57,69,105, 149,163, 207 |
Anticancer, antiviral |
1.11. Nutritional Values of Different Parts of the Plant
The investigation of the dietary arrangement of each one of those parts is necessary. The plant pieces and corresponding nutritional values are given in Table 9. Besides, Table 10 summarizes the sugar content in different parts of M. sylvestris.
Table 9.
Energetic value (Kcal/100 g of dry weight), macronutrients' composition (g/100 g of dry weight), and moisture (g/100 g of fresh weight) of different M. sylvestris components [2].
| Leafy flowered | Immature fruits | Flowers | Leaves | |
|---|---|---|---|---|
| Energy | 372.43 ± 1.08 b | 393.45 ± 4.41 a | 372.02 ± 2.13 b | 359.72 ± 1.10 c |
| Reduced sugars | 10.46 ± 0.70 b | 2.09 ± 0.12 d | 13.95 ± 0.16 a | 6.22 ± 0.49 c |
| Ash | 10.76 ± 0.04 b | 12.83 ± 0.78 a | 10.54 ± 0.30 b | 13.53 ± 0.11 a |
| Fat | 3.09 ± 0.27 b | 8.96 ± 0.22 a | 2.84 ± 0.37 b | 2.76 ± 0.40 b |
| Proteins | 14.26 ± 0.44 a | 3.26 ± 0.25 d | 8.50 ± 0.51 c | 12.25 ± 1.01 b |
| Carbohydrates | 71.89 ± 0.35 c | 74.96 ± 1.10 b | 78.12 ± 0.44 a | 71.46 ± 0.81 c |
| Moisture | 77.26 ± 1.34 a | 45.60 ± 0.97 d | 72.49 ± 1.89 c | 76.30 ± 0.54 b |
Table 10.
Sugar composition (g/100 g of dry weight) of numerous M. sylvestris components (mean ± SD; n = 3).
| Leafy flowered stems | Immature fruits | Flowers | Leaves | |
|---|---|---|---|---|
| Raffinose | Nd | 0.26 ± 0.03 a | Nd | Nd |
| Trehalose | 3.09 ± 0.03 a | Nd | 1.47 ± 0.06 c | 2.67 ± 0.11 b |
| Sucrose | 3.30 ± 0.10 a | 0.11 ± 0.03 d | 2.47 ± 0.05 c | 3.97 ± 0.03 b |
| Glucose | 4.74 ± 0.18 b | 1.52 ± 0.07 d | 7.36 ± 0.13 a | 3.15 ± 0.43 c |
| Fructose | 3.53 ± 0.18 b | 0.40 ± 0.03 d | 8.72 ± 0.14 a | 1.82 ± 0.23 c |
| Total sugars | 14.67 ± 0.49 b | 2.30 ± 0.10 d | 20.02 ± 0.26 a | 11.61 ± 0.51 c |
In each row, different letters mean significant differences (p < 0.05) [2].
Gas chromatography-mass spectrometry (GC-MS) evaluation is a practical approach used for countless functions with the most excellent sensitivity and specificity. A volume of 1 μL methanol extract of Malva sylvestris was infused into the GC-MS and inspected typically for 45 minutes. The period since the infusion was made (initial time) to when washing occurred is referred to as the retention time (RT) [81, 82]. Helium fuel containing an eluent was used as a carrier [83].
1.12. Antioxidant Activity
M. sylvestris has antiradical properties due to high phenolic contents and is capable of preventing oxidation. Flavenoid compounds in this plant have high inhibitory power. These plants are also free of complications in comparison to chemical drugs [84]. The production of different oxygen species over the body's antioxidants causes oxidative stress. Evidence suggests that stress is one of the essential factors of aging in brain function, liver disease, cardiovascular disorders, and cancer [85].
1.13. Anti-Inflammatory Activity
Several research groups have investigated M. sylvestris anti-inflammatory activity [35]. Their results support the notion that the compound malvidin 3-glucoside seems to be primarily accountable for this effect, and M. sylvestris leaves possess topical anti-inflammatory properties. The results of studies on the antimicrobial properties of M. sylvestris indicate that the plant also has antibacterial and antiviral activity against many human pathogens [86].
1.14. Anticancer Activity
Cancer is a generic term for a significant group of diseases that can affect any part of the body. Based on the report of World Health Organization (WHO), cancer is a leading cause of death universally. Reports show that M. sylvestris possesses anticancer properties. Daniela et al. [41] demonstrated cytotoxic activity of M. sylvestris leaf extracts on murine using an MTT assay and human cancer cell lines. The biological test found that M. sylvestris extracts significantly decrease cancer cell lines (Figure 5) [5, 41, 87, 88].
Figure 5.
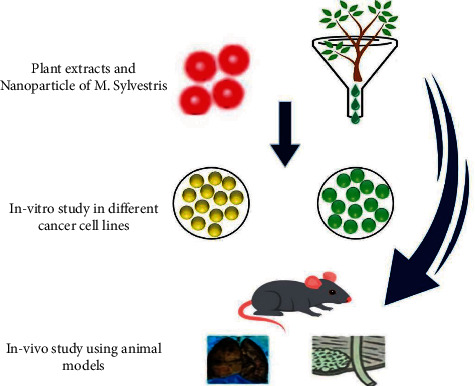
In vivo and in vitro anticancer activities of M. sylvestris against different types of cancer [41].
1.15. Wound Healing Activity
The topical application of the ethanolic hydroalcoholic extract of Malva leaves in a dose-dependent manner increases the rate of contraction of skin ulcers and reduces the duration of its repair process in rats. On the other hand, fiber plants are responsible for producing and secreting collagen. Protein collagens are a central extracellular matrix, which leads to an increase in the ability of wound edges to bind to each other.
1.16. Hepatoprotective Activity
The liver should be physiologically involved in all vital functions of the body. Any malfunction in the liver causes a set of disorders that can cause irreparable damage to this member; influential factors such as oxidative stress, free radicals, chemicals, viruses, and medicines can cause liver tissue degradation [89–91]. The literature confirmed the presence of antioxidant compounds in M. sylvestris. These compounds, in turn, remove the free radicals and help protect tissues, especially in the liver [92].
1.17. Antiosteoporosis Activity
Because of the imbalance between osteoblast and osteoclast activities, osteoporosis leads to weakening bone strength and elevation of fracture risk [93, 94]. M. sylvestris aqueous extracts can induce the activity of the signaling pathways and affect the osteoblast in an osteoclast difference [12, 86].
1.18. Antinociceptive Activity
The antinociceptive activity of M. sylvestris aqueous extracts was assessed against traditional pain models in mice by Esteves et al. [10]. Extensive antinociceptive activity was demonstrated in the writhing test (76.4% of inhibition), as well as inhibition of inflammation (46.6%) and neurogenic (61.8%) phases of the formalin model. Their outcomes suggest that M. sylvestris possesses stimulating substances, which act as antinociceptive agents.
1.19. Antimicrobial Activity
M. sylvestris performs antimicrobial activities against various bacterial and fungal species. The disc diffusion method has reported the antimicrobial activity of M. sylvestris extracts against different bacterial species. The researchers found that M. sylvestris has moderate activity against selected microorganisms associated with typical antibiotics [95].
De Souza et al. [96] studied the antimicrobial activity of M. sylvestris aerial part extracts against C. Albicans, S. aureus, M. luteus, Bacillus subtilis, S. epidermidis, E. coli, and S. cerevisiae [97]. Their study reported that ethanol extracts of M. sylvestris were active against P. aeruginosa, B. subtilis, and E. coli, whereas methanol extracts showed activity only against S. cerevisiae [98]. Their results demonstrated that M. sylvestris extracts inhibited the in vitro microbial activity. Other studies showed that the seed oil inhibits the growth of all microorganisms tested except the Gram-negative bacteria P. aeruginosa [99–101].
1.20. Preventive Effect of M. sylvestris on Urinary Toxicity after Radiation Therapy in Prostate Cancer
M. sylvestris has a preventive effect on urinary toxicity after radiation therapy in prostate cancer in terms of relieving the pain related to external beam radiation therapy- (EBRT-) induced urinary toxicity. Up-to-date radiotherapy techniques, for instance, three-dimensional conformal radiation therapy (3D-CRT) and intensity-modulated radiation therapy (IMRT), can reduce genitourinary and gastrointestinal toxicity induced by EBRT [102].
1.21. Antifungal Assay
The antifungal activities of the plant extracts were the same against Penicillium spp., C. Albicans, Aspergillus niger, Candida kefir, and Sclerotinia sclerotiorum by the circle dissemination technique. Amphotericin B (10 µg) was considered a positive control, and the plates were cultured at 30°C for 48 hours. The minimal inhibitory concentrations (MICs) of the concentrates against the test microorganisms were controlled by the agar diffusion strategy [3, 103, 104].
1.22. Healing of Atopic Dermatitis
M. sylvestris is the most common dermatological ailment treatment, for example, atopic dermatitis; however, conventional therapeutics, such as corticosteroids and antihistamines, have no effects [105]. Natural agents, which generally have no extensive side effects, could be used to determine its efficacy. In this study, its effectiveness in treating atopic dermatitis was assessed and it could topically be used as an effective cream to reduce the dermatitis symptoms in children.
2. Conclusion
This review showed the significance of M. sylvestris as a medicinal herb and functional food. Findings indicate that relatively extensive research has been carried out on chemical compounds and pharmacological effects, as well as different aspects of the Malva plant. M. sylvestris is an important resourceful plant because of its effective medicinal properties. Studies have proven its potential for health benefits due to its antioxidant activity, anti-inflammatory activity, anticancer activity, wound-healing activity, hepatoprotective activity, antinociceptive activity, and antimicrobial activity. The leaves, flowers, and roots are used for medicinal reasons. Herein, one-of-its-kind organic activities of M. sylvestris L., traditional uses, main phytochemical compounds detected in methanolic extracts, and pharmacological activities of M. sylvestris were reviewed.
Data Availability
All the data used to support the findings of this study are included within the article.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Alizadeh F., Khodavandi A. Malva sylvestris inhibits Candida albicans biofilm formation. Journal of Herbmed Pharmacology. 2016;6 [Google Scholar]
- 2.Barros L., Carvalho A. M., Ferreira I. C. F. R. Leaves, flowers, immature fruits and leafy flowered stems of Malva sylvestris: a comparative study of the nutraceutical potential and composition. Food and Chemical Toxicology. 2010;48(6):1466–1472. doi: 10.1016/j.fct.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 3.Razavi S. M., Zarrini G., Molavi G., Ghasemi G. Bioactivity of Malva sylvestris L., a medicinal plant from Iran. Iranian journal of basic medical sciences. 2011;14(6):574–9. [PMC free article] [PubMed] [Google Scholar]
- 4.Guarrera P. M. Traditional phytotherapy in Central Italy (Marche, Abruzzo, and latium) Fitoterapia. 2005;76(1):1–25. doi: 10.1016/j.fitote.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 5.Mazraedoost S., Behbudi G. Nano materials-based devices by photodynamic therapy for treating cancer applications. Advances in Applied NanoBio-Technologies. 2021;2(3):9–21. [Google Scholar]
- 6.Pirbalouti A. G., Azizi S., Koohpayeh A., Hamedi B. Wound healing activity of Malva sylvestris and Punica granatum in alloxan-induced diabetic rats. Acta Poloniae Pharmaceutica. 2010;67(5):511–516. [PubMed] [Google Scholar]
- 7.Gasparetto J. C., Martins C. A. F., Hayashi S. S., Otuky M. F., Pontarolo R. Ethnobotanical and scientific aspects of Malva sylvestris L.: a millennial herbal medicine. Journal of Pharmacy and Pharmacology. 2012;64(2):172–189. doi: 10.1111/j.2042-7158.2011.01383.x. [DOI] [PubMed] [Google Scholar]
- 8.Farina A., Doldo A., Cotichini V., et al. HPTLC and reflectance mode densitometry of anthocyanins in Malva silvestris L.: a comparison with gradient-elution reversed-phase HPLC. Journal of Pharmaceutical and Biomedical Analysis. 1995;14(1-2):203–211. doi: 10.1016/0731-7085(95)01632-5. [DOI] [PubMed] [Google Scholar]
- 9.Yeole N., Sandhya P., Chaudhari P., Bhujbal P. Evaluation of Malva sylvestris and Pedalium murex mucilage as suspending agent. International Journal of PharmTech Research. 2010;2(1):385–389. [Google Scholar]
- 10.Esteves P. F., Sato A., Esquibel M. A., De Campos-Buzzi F., Meira A. V., Cechinel-Filho V. Antinociceptive activity of Malva sylvestris L. Latin American Journal of Pharmacy. 2009;28(3):454–456. [Google Scholar]
- 11.Ana Maria C. Etnobotánica del Parque Natural de Montesinho Plantas, Tradición y Saber Popular en un Territorio del Nordeste de Portugal. Madrid, Spain: Universidad Autónoma de Madrid; 2005. [Google Scholar]
- 12.Benso B., Franchin M., Massarioli A. P., et al. Anti-inflammatory, anti-osteoclastogenic and antioxidant effects of Malva sylvestris extract and fractions: in vitro and in vivo studies. PLoS One. 2016;11(9):p. e0162728. doi: 10.1371/journal.pone.0162728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martins C., Campos M., Irioda A., Stremel D., Trindade A., Pontarolo R. Anti-inflammatory effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens is related to inhibition of prostanoid production. Molecules. 2017;22(11):p. 1883. doi: 10.3390/molecules22111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mousavi S. M., Hashemi S. A., Zarei M., et al. Data on cytotoxic and antibacterial activity of synthesized Fe3O4 nanoparticles using Malva sylvestris. Data in brief. 2020;28:p. 104929. doi: 10.1016/j.dib.2019.104929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gholami A., Shahin S., Mohkam M., Nezafat N., Ghasemi Y. Cloning, characterization and bioinformatics analysis of novel cytosine deaminase from Escherichia coli AGH09. International Journal of Peptide Research and Therapeutics. 2015;21(3):365–374. doi: 10.1007/s10989-015-9465-9. [DOI] [Google Scholar]
- 16.Mohamadi Yarijani Z., Najafi H., Shackebaei D., Madani S. H., Modarresi M., Jassemi S. V. Amelioration of renal and hepatic function, oxidative stress, inflammation and histopathologic damages by Malva sylvestris extract in gentamicin induced renal toxicity. Biomedicine & Pharmacotherapy. 2019;112:p. 108635. doi: 10.1016/j.biopha.2019.108635. [DOI] [PubMed] [Google Scholar]
- 17.Wichtl M. Herbal Drugs and Phytopharmaceuticals. Boca Roton, FL, USA: CRC Press; 1994. [Google Scholar]
- 18.Veshkurova O., Golubenko Z., Pshenichnov E., et al. Malvone A, a phytoalexin found in Malva sylvestris (family Malvaceae) Phytochemistry. 2006;67(21):2376–2379. doi: 10.1016/j.phytochem.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 19.Nawwar M. A. M., Buddrus J. A gossypetin glucuronide sulphate from the leaves of Malva sylvestris. Phytochemistry. 1981;20(10):2446–2448. doi: 10.1016/s0031-9422(00)82694-1. [DOI] [Google Scholar]
- 20.Terninko I. I., Onishchenko U. E. Component composition of organic acids in leaves of Malva sylvestris. Chemistry of Natural Compounds. 2013;49(2):332–333. doi: 10.1007/s10600-013-0594-0. [DOI] [Google Scholar]
- 21.Petkova N., Popova A., Alexieva I. Antioxidant properties and some phytochemical components of the edible medicinal Malva sylvestris L. Journal of Medicinal Plants. 2019;7(1):96–99. [Google Scholar]
- 22.Toledo M. D. G. T. D., Alquini Y., Nakashima T. Caracterização anatômica das folhas de Cunila microcephala Benth. (Lamiaceae) Revista Brasileira de Ciencias Farmaceuticas. 2004;40(4):487–493. doi: 10.1590/s1516-93322004000400006. [DOI] [Google Scholar]
- 23.Redžić S., Hodžić N., Tuka M. Plant pigments (antioxidants) of medicinal plants Malva sylvestris L. and Malva moschata L.(Malvaceae) Bosnian Journal of Basic Medical Sciences. 2005;5(2):53–58. doi: 10.17305/bjbms.2005.3284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hiçsönmez U., Ereeş F. S., Özdemir C., Özdemir A., Cam S. Determination of major and minor elements in the Malva sylvestris L. from Turkey using ICP-OES techniques. Biological Trace Element Research. 2009;128(3):248–257. doi: 10.1007/s12011-008-8270-0. [DOI] [PubMed] [Google Scholar]
- 25.Desideri D., Meli M. A., Roselli C. Determination of essential and non-essential elements in some medicinal plants by polarised X ray fluorescence spectrometer (EDPXRF) Microchemical Journal. 2010;95(2):174–180. doi: 10.1016/j.microc.2009.11.010. [DOI] [Google Scholar]
- 26.Najafi H., Mohamadi Yarijani Z., Changizi-Ashtiyani S., et al. Protective effect of Malva sylvestris L. extract in ischemia-reperfusion induced acute kidney and remote liver injury. PLoS One. 2017;12(11):p. e0188270. doi: 10.1371/journal.pone.0188270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ganai B. A., Masood A., Baig M. A. Isolation, purification and partial characterization of sulphite oxidase from Malva sylvestris. Phytochemistry. 1997;45(5):879–880. doi: 10.1016/s0031-9422(97)82709-4. [DOI] [Google Scholar]
- 28.Abootalebi S. N., Saeed A., Gholami A., et al. Screening, characterization and production of thermostable alpha-amylase produced by a novel thermophilic Bacillus megaterium isolated from pediatric intensive care unit. Journal of Environmental Treatment Techniques. 2020;8(3):952–960. [Google Scholar]
- 29.Gholami A., Mohammadi F., Ghasemi Y., Omidifar N., Ebrahiminezhad A. Antibacterial activity of SPIONs versus ferrous and ferric ions under aerobic and anaerobic conditions: a preliminary mechanism study. IET Nanobiotechnology. 2019;14(2):155–160. doi: 10.1049/iet-nbt.2019.0266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Willey N. J., Fawcett K. Inter-taxa differences in root uptake of 103/106Ru by plants. Journal of Environmental Radioactivity. 2006;86(2):227–240. doi: 10.1016/j.jenvrad.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 31.Goudarzian N., Sadeghi Z., Mousavi S. M., Hashemi S. A., Banaei N. Chemical constituent and determination of antimicrobial and antifungal activities of Ulva lactuca species obtained from Iranian Gheshm Island. International Journal of Scientific Engineering and Research. 2017;8:1275–1279. [Google Scholar]
- 32.Gholami A., Rasoul-Amini S., Ebrahiminezhad A., et al. Magnetic properties and antimicrobial effect of amino and lipoamino acid coated iron oxide nanoparticles. Minerva Biotecnologica. 2016;28(4):177–186. [Google Scholar]
- 33.Ahmadi S. The importance of silver nanoparticles in human life. Advances in Applied NanoBio-Technologies. 2020;1(1):5–9. doi: 10.47277/aanbt/1(1)9. [DOI] [Google Scholar]
- 34.Magro A., Carolino M., Bastos M., Mexia A. Efficacy of plant extracts against stored products fungi. Revista Iberoamericana De Micologia. 2006;23(3):176–178. doi: 10.1016/s1130-1406(06)70039-0. [DOI] [PubMed] [Google Scholar]
- 35.Sleiman N., Daher C. Malva sylvestris water extract: a potential anti-inflammatory and anti-ulcerogenic remedy. Planta Medica. 2009;75(9):p. PH10. doi: 10.1055/s-0029-1234727. [DOI] [Google Scholar]
- 36.Mohkam M., Rasoul-Amini S., Shokri D., et al. Characterization and in vitro probiotic assessment of potential indigenous Bacillus strains isolated from soil rhizosphere. Minerva Biotechnologica. 2016;28(1):19–28. [Google Scholar]
- 37.Wilson C. R. Incidence of weed reservoirs and vectors of tomato spotted wilt tospovirus on Southern Tasmanian lettuce farms. Plant Pathology. 1998;47(2):171–176. doi: 10.1046/j.1365-3059.1998.00227.x. [DOI] [Google Scholar]
- 38.Schmidgall J., Schnetz E., Hensel A. Evidence for bioadhesive effects of polysaccharides and polysaccharide-containing herbs in an ex vivo bioadhesion assay on buccal membranes. Planta Medica. 2000;66(1):48–53. doi: 10.1055/s-2000-11118. [DOI] [PubMed] [Google Scholar]
- 39.Talbourdet S., Sadick N. S., Lazou K., et al. Modulation of gene expression as a new skin anti-aging strategy. Journal of Drugs in Dermatology: Journal of Drugs in Dermatology. 2007;6(6 Suppl):s25–33. [PubMed] [Google Scholar]
- 40.Kumarasamy Y., Byres M., Cox P. J., Jaspars M., Nahar L., Sarker S. D. Screening seeds of some Scottish plants for free radical scavenging activity. Phytotherapy Research. 2007;21(7):615–621. doi: 10.1002/ptr.2129. [DOI] [PubMed] [Google Scholar]
- 41.Daniela A., Pichichero E., Canuti L., et al. Identification of phenolic compounds from medicinal and melliferous plants and their cytotoxic activity in cancer cells. Caryologia. 2007;60(1-2):90–95. doi: 10.1080/00087114.2007.10589552. [DOI] [Google Scholar]
- 42.Yagi A. 14 bioactivity of aloe arborescens preparations. Aloes: The genus Aloe. 2004;333 [Google Scholar]
- 43.Scherrer A. M., Motti R., Weckerle C. S. Traditional plant use in the areas of monte vesole and ascea, cilento national park (Campania, Southern Italy) Journal of Ethnopharmacology. 2005;97(1):129–143. doi: 10.1016/j.jep.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 44.Motti R., Antignani V., Idolo M. Traditional plant use in the phlegraean fields regional park (Campania, Southern Italy) Human Ecology. 2009;37(6):775–782. doi: 10.1007/s10745-009-9254-1. [DOI] [Google Scholar]
- 45.Miraldi E., Ferri S., Mostaghimi V. Botanical drugs and preparations in the traditional medicine of West Azerbaijan (Iran) Journal of Ethnopharmacology. 2001;75(2-3):77–87. doi: 10.1016/s0378-8741(00)00381-0. [DOI] [PubMed] [Google Scholar]
- 46.Ishtiaq M., Hanif W., Khan M. A., Ashraf M., Butt A. M. An ethnomedicinal survey and documentation of important medicinal folklore food phytonims of flora of samahni valley, (Azad kashmir) Pakistan. Pakistan Journal of Biological Sciences. 2007;10(13):2241–2256. doi: 10.3923/pjbs.2007.2241.2256. [DOI] [PubMed] [Google Scholar]
- 47.González-Tejero M. R., Casares-Porcel M., Sánchez-Rojas C. P., et al. Medicinal plants in the Mediterranean area: synthesis of the results of the project Rubia. Journal of Ethnopharmacology. 2008;116(2):341–357. doi: 10.1016/j.jep.2007.11.045. [DOI] [PubMed] [Google Scholar]
- 48.Al-Qura’n S. Ethnopharmacological survey of wild medicinal plants in Showbak, Jordan. Journal of Ethnopharmacology. 2009;123(1):45–50. doi: 10.1016/j.jep.2009.02.031. [DOI] [PubMed] [Google Scholar]
- 49.Rehecho S., Uriarte-Pueyo I., Calvo J., Vivas L. A., Calvo M. I. Ethnopharmacological survey of medicinal plants in nor-yauyos, a part of the landscape reserve nor-yauyos-cochas, Peru. Journal of Ethnopharmacology. 2011;133(1):75–85. doi: 10.1016/j.jep.2010.09.006. [DOI] [PubMed] [Google Scholar]
- 50.Joshi R., Satyal P., Setzer W. Himalayan aromatic medicinal plants: a review of their ethnopharmacology, volatile phytochemistry, and biological activities. Medicines. 2016;3(1):p. 6. doi: 10.3390/medicines3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Leonti M., Casu L., Sanna F., Bonsignore L. A comparison of medicinal plant use in Sardinia and Sicily-De materia medica revisited? Journal of Ethnopharmacology. 2009;121(2):255–267. doi: 10.1016/j.jep.2008.10.027. [DOI] [PubMed] [Google Scholar]
- 52.Touwaide A., De Santo N. G., Aliotta G. The origins of Western herbal medicines for kidney diseases. Advances in Chronic Kidney Disease. 2005;12(3):251–260. doi: 10.1016/j.ackd.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 53.Nelly A., Annick D.-D., Frederic D. Plants used as remedies antirheumatic and antineuralgic in the traditional medicine of Lebanon. Journal of Ethnopharmacology. 2008;120(3):315–334. doi: 10.1016/j.jep.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 54.Ballero M., Poli F., Sacchetti G., Loi M. C. Ethnobotanical research in the territory of Fluminimaggiore (South-Western Sardinia) Fitoterapia. 2001;72(7):788–801. doi: 10.1016/s0367-326x(01)00334-3. [DOI] [PubMed] [Google Scholar]
- 55.Pieroni A., Nebel S., Quave C., Münz H., Heinrich M. Ethnopharmacology of liakra: traditional weedy vegetables of the Arbëreshë of the Vulture area in Southern Italy. Journal of Ethnopharmacology. 2002;81(2):165–185. doi: 10.1016/s0378-8741(02)00052-1. [DOI] [PubMed] [Google Scholar]
- 56.De Natale A., Pollio A. Plants species in the folk medicine of Montecorvino Rovella (Inland Campania, Italy) Journal of Ethnopharmacology. 2007;109(2):295–303. doi: 10.1016/j.jep.2006.07.038. [DOI] [PubMed] [Google Scholar]
- 57.Guarrera P. M., Salerno G., Caneva G. Folk phytotherapeutical plants from Maratea area (Basilicata, Italy) Journal of Ethnopharmacology. 2005;99(3):367–378. doi: 10.1016/j.jep.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 58.Loi M. C., Poli F., Sacchetti G., Selenu M. B., Ballero M. Ethnopharmacology of ogliastra (Villagrande Strisaili, Sardinia, Italy) Fitoterapia. 2004;75(3-4):277–295. doi: 10.1016/j.fitote.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 59.Kültür Ş. Medicinal plants used in Kırklareli Province (Turkey) Journal of Ethnopharmacology. 2007;111(2):341–364. doi: 10.1016/j.jep.2006.11.035. [DOI] [PubMed] [Google Scholar]
- 60.Benítez G., González-Tejero M. R., Molero-Mesa J. Pharmaceutical ethnobotany in the western part of Granada province (southern Spain): ethnopharmacological synthesis. Journal of Ethnopharmacology. 2010;129(1):87–105. doi: 10.1016/j.jep.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 61.Idolo M., Motti R., Mazzoleni S. Ethnobotanical and phytomedicinal knowledge in a long-history protected area, the Abruzzo, lazio and molise national park (Italian Apennines) Journal of Ethnopharmacology. 2010;127(2):379–395. doi: 10.1016/j.jep.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 62.González J. A., García-Barriuso M., Amich F. Ethnobotanical study of medicinal plants traditionally used in the Arribes del Duero, Western Spain. Journal of Ethnopharmacology. 2010;131(2):343–355. doi: 10.1016/j.jep.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 63.Leporatti M. L., Ivancheva S. Preliminary comparative analysis of medicinal plants used in the traditional medicine of Bulgaria and Italy. Journal of Ethnopharmacology. 2003;87(2-3):123–142. doi: 10.1016/s0378-8741(03)00047-3. [DOI] [PubMed] [Google Scholar]
- 64.Ameri A., Heydarirad G., Mahdavi Jafari J., Ghobadi A., Rezaeizadeh H., Choopani R. Medicinal plants contain mucilage used in traditional Persian medicine (TPM) Pharmaceutical Biology. 2015;53(4):615–623. doi: 10.3109/13880209.2014.928330. [DOI] [PubMed] [Google Scholar]
- 65.Ghorbani A., Aghjeh Qeshlagh M., Karamati Jabehdar S. Folk herbal veterinary medicines of Tehran Watershed (Iran) Journal of Herbal Drugs (An International Journal on Medicinal Herbs) 2015;6(1):31–39. [Google Scholar]
- 66.Azab A. Malva: food, medicine and chemistry. European Chemical Bulletin. 2017;6(7):295–320. doi: 10.17628/ecb.2017.6.295-320. [DOI] [Google Scholar]
- 67.Boubakr S., Ali L., Zoheir M., Zahra H., Mohamed D., Boukeur A. Global journal of medicinal plant research. Global Journal of Medicinal Plant Research. 2015;3(5):1–16. [Google Scholar]
- 68.Bulut G., Tuzlacı E. An ethnobotanical study of medicinal plants in Bayrami̇ç. Marmara Pharmaceutical Journal. 2015;19(3):268–282. doi: 10.12991/mpj.201519392830. [DOI] [Google Scholar]
- 69.Mayer M., Vogl C. R., Amorena M., Hamburger M., Walkenhorst M. Treatment of organic livestock with medicinal plants: a systematic review of European ethnoveterinary research. Complementary Medicine Research. 2014;21(6):375–386. doi: 10.1159/000370216. [DOI] [PubMed] [Google Scholar]
- 70.Menale B., Muoio R. Use of medicinal plants in the south-eastern area of the partenio regional park (Campania, Southern Italy) Journal of Ethnopharmacology. 2014;153(1):297–307. doi: 10.1016/j.jep.2014.02.039. [DOI] [PubMed] [Google Scholar]
- 71.Toklu H. Z. Herbal medicine use among diabetes mellitus patients in Northern Cyprus. Journal of Medicinal Plants Research. 2013;7(22):1652–1664. [Google Scholar]
- 72.Javed A., Usman M., Haider S. M., Zafar B., Iftikhar K. Potential of indigenous plants for skin healing and care. American Scientific Research Journal for Engineering, Technology, and Sciences (ASRJETS) 2019;51(1):192–211. [Google Scholar]
- 73.Hassan G., Ahmad T., Mohi-Ud-Din R. An ethnobotanical study in Budgam district of Kashmir valley: an attempt to explore and document traditional knowledge of the area. International Research Journal of Pharmacy. 2013;4:201–204. [Google Scholar]
- 74.Kumar N., Wani Z., Dhyani S. Ethnobotanical study of the plants used by the local people of Gulmarg and its allied areas, Jammu & Kashmir, India. International Journal of Current Research in Biosciences and Plant Biology. 2015;2(9):16–23. [Google Scholar]
- 75.Bieski I. G. C., Rios Santos F., De Oliveira R. M., et al. Ethnopharmacology of medicinal plants of the pantanal region (Mato Grosso, Brazil) Evidence-Based Complementary and Alternative Medicine. 2012;2012 doi: 10.1155/2012/272749.272749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luczaj L. Ethnobotanical review of wild edible plants of Slovakia. Acta Societatis Botanicorum Poloniae. 2012;81(4) [Google Scholar]
- 77.Alachkar A., Jaddouh A., Elsheikh M. S., Bilia A. R., Vincieri F. F. Traditional medicine in Syria: folk medicine in Aleppo governorate. Natural Product Communications. 2011;6(1):p. 84. doi: 10.1177/1934578x1100600119. [DOI] [PubMed] [Google Scholar]
- 78.Parada M., Carrió E., Vallès J. Ethnobotany of food plants in the Alt emporda region (Catalonia, Iberian Peninsula) Journal of Applied Botany and Food Quality. 2011;84(1):11–25. [Google Scholar]
- 79.Ouhaddou H., Boubaker H., Msanda F., El Mousadik A. An ethnobotanical study of medicinal plants of the Agadir Ida Ou Tanane province (Southwest Morocco) Journal of Applied Biosciences. 2014;84(1):7707–7722. [Google Scholar]
- 80.Kappelle M., Avertin G., Juárez M. E., Zamora N. Useful plants within a campesino community in a Costa Rican montane cloud forest. Mountain Research and Development. 2000;20(2):162–171. doi: 10.1659/0276-4741(2000)020[0162:upwacc]2.0.co;2. [DOI] [Google Scholar]
- 81.Altaee N., Kadhim M. J., Hameed I. H. Characterization of metabolites produced by E. coli and analysis of its chemical compounds using GC-MS. International Journal of Current Pharmaceutical Review and Research. 2017;7(6):13–19. [Google Scholar]
- 82.Hadi M. Y., Hameed I. H. Uses of gas chromatography-mass spectrometry (GC-MS) technique for analysis of bioactive chemical compounds of lepidium sativum: a review. Research Journal of Pharmacy and Technology. 2017;10(11):4039–4042. doi: 10.5958/0974-360x.2017.00732.6. [DOI] [Google Scholar]
- 83.Jawad Kadhim M., Mohammed G. J., Hadi Hameed I. In vitro antibacterial, antifungal and phytochemical analysis of methanolic extract of fruit Cassia fistula. Oriental Journal of Chemistry. 2016;32(3):1329–1346. doi: 10.13005/ojc/320307. [DOI] [Google Scholar]
- 84.Marouane W., Soussi A., Murat J.-C., Bezzine S., El Feki A. The protective effect of Malva sylvestris on rat kidney damaged by vanadium. Lipids in Health and Disease. 2011;10(1):p. 65. doi: 10.1186/1476-511x-10-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jaradat N., Abualhasan M., Ali I. Comparison of anti-oxidant activities and exhaustive extraction yields between wild and cultivated cyclamen persicum, Malva sylvestris and Urtica pilulifera leaves. Journal of Applied Pharmaceutical Science. 2015;5(4):101–106. doi: 10.7324/japs.2015.50417. [DOI] [Google Scholar]
- 86.Benso B., Rosalen P. L., Alencar S. M., Murata R. M. Malva sylvestris inhibits inflammatory response in oral human cells. An in vitro infection model. PLoS One. 2015;10(10):p. e0140331. doi: 10.1371/journal.pone.0140331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Abbaszadegan A., Gholami A., Ghahramani Y., et al. Antimicrobial and cytotoxic activity of cuminum cyminum as an intracanal medicament compared to chlorhexidine gel. Iranian Endodontic Journal. 2016;11(1):44–50. doi: 10.7508/iej.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mazraedoost S., Behbudi G. Basic nano magnetic particles and essential oils: biological applications. Journal of Environmental Treatment Techniques. 2021;9(3):609–620. [Google Scholar]
- 89.Azarang A., Farshad O., Ommati M. M., et al. Protective role of probiotic supplements in hepatic steatosis: a rat model study. BioMed Research International. 2020;2020 doi: 10.1155/2020/5487659.5487659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Omidifar N., Nili-Ahmadabadi A., Gholami A., Dastan D., Ahmadimoghaddam D., Nili-Ahmadabadi H. Biochemical and histological evidence on the protective effects of Allium hirtifolium boiss (Persian shallot) as an herbal supplement in cadmium-induced hepatotoxicity. Evidence-Based Complementary and Alternative Medicine: ECAM. 2020;2020 doi: 10.1155/2020/7457504.7457504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Gholami A., Emadi F., Nazem M., et al. Expression of key apoptotic genes in hepatocellular carcinoma cell line treated with etoposide-loaded graphene oxide. Journal of Drug Delivery Science and Technology. 2020;57:p. 101725. doi: 10.1016/j.jddst.2020.101725. [DOI] [Google Scholar]
- 92.Hussain L., Ikram J., Rehman K., Tariq M., Ibrahim M., Akash M. S. H. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turkish Journal of Biology. 2014;38(3):396–402. doi: 10.3906/biy-1312-32. [DOI] [Google Scholar]
- 93.Montazeri-Najafabady N., Ghasemi Y., Dabbaghmanesh M. H., et al. Exploring the bone sparing effects of postbiotics in the post-menopausal rat model. BMC Complementary Medicine and Therapies. 2021;21(1):p. 155. doi: 10.1186/s12906-021-03327-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Montazeri-Najafabady N., Ghasemi Y., Dabbaghmanesh M. H., Talezadeh P., Koohpeyma F., Gholami A. Supportive role of probiotic strains in protecting rats from ovariectomy-induced cortical bone loss. Probiotics and Antimicrobial Proteins. 2019;11(4):1145–1154. doi: 10.1007/s12602-018-9443-6. [DOI] [PubMed] [Google Scholar]
- 95.Dulger B., Gonuz A. Antimicrobial activity of certain plants used in Turkish traditional medicine. Asian Journal of Plant Sciences. 2004;3(1):104–107. [Google Scholar]
- 96.Coelho De Souza G., Haas A. P. S., Von Poser G. L., Schapoval E. E. S., Elisabetsky E. Ethnopharmacological studies of antimicrobial remedies in the south of Brazil. Journal of Ethnopharmacology. 2004;90(1):135–143. doi: 10.1016/j.jep.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 97.Cogo L. L., Monteiro C. L. B., Miguel M. D., et al. Anti-Helicobacter pylori activity of plant extracts traditionally used for the treatment of gastrointestinal disorders. Brazilian Journal of Microbiology. 2010;41(2):304–309. doi: 10.1590/s1517-83822010000200007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng C.-L., Wang Z.-Y. Bacteriostasic activity of anthocyanin of Malva sylvestris. Journal of Forestry Research. 2006;17(1):83–85. doi: 10.1007/s11676-006-0020-6. [DOI] [Google Scholar]
- 99.Walter C., Shinwari Z. K., Afzal I., Malik R. N. Antibacterial activity in herbal products used in Pakistan. Pakistan Journal of Botany. 2011;43:155–162. [Google Scholar]
- 100.Mousavi S. M., Hashemi S. A., Zarei M., et al. Recent progress in chemical composition, production, and pharmaceutical effects of kombucha beverage: a complementary and alternative medicine. Evidence-Based Complementary and Alternative Medicine. 2020;2020 doi: 10.1155/2020/4397543.4397543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gholami A., Hashemi S. A., Yousefi K., et al. 3D nanostructures for tissue engineering, cancer therapy, and gene delivery. Journal of Nanomaterials. 2020;2020 doi: 10.1155/2020/1852946.1852946 [DOI] [Google Scholar]
- 102.Razavi S. M., Zarrini G. Bioactivity of aviprin and aviprin-3″-O-glucoside, two linear furanocoumarins from Apiaceae. Russian Journal of Bioorganic Chemistry. 2010;36(3):359–362. doi: 10.1134/s1068162010030118. [DOI] [PubMed] [Google Scholar]
- 103.Nabavizadeh M., Abbaszadegan A., Gholami A, et al. Chemical constituent and antimicrobial effect of essential oil from Myrtus communis leaves on microorganisms involved in persistent endodontic infection compared to two common endodontic irrigants: an in vitro study. Journal of Conservative Dentistry: Journal of Computational Dynamics. 2014;17(5):449–453. doi: 10.4103/0972-0707.139836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abbaszadegan A., Sahebi S., Gholami A., et al. Time-dependent antibacterial effects of aloe vera and Zataria multiflora plant essential oils compared to calcium hydroxide in teeth infected with Enterococcus faecalis. Journal of Investigative and Clinical Dentistry. 2016;7(1):93–101. doi: 10.1111/jicd.12123. [DOI] [PubMed] [Google Scholar]
- 105.Meysami M., Hashempur M. H., Kamalinejad M., Emtiazy M. Efficacy of short term topical Malva sylvestris L. Cream in pediatric patients with atopic dermatitis: a randomized double-blind PlaceboControlled clinical trial. Endocrine, Metabolic & Immune Disorders-Drug Targets. 2020;20 doi: 10.2174/1871530320666201023125411. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All the data used to support the findings of this study are included within the article.


