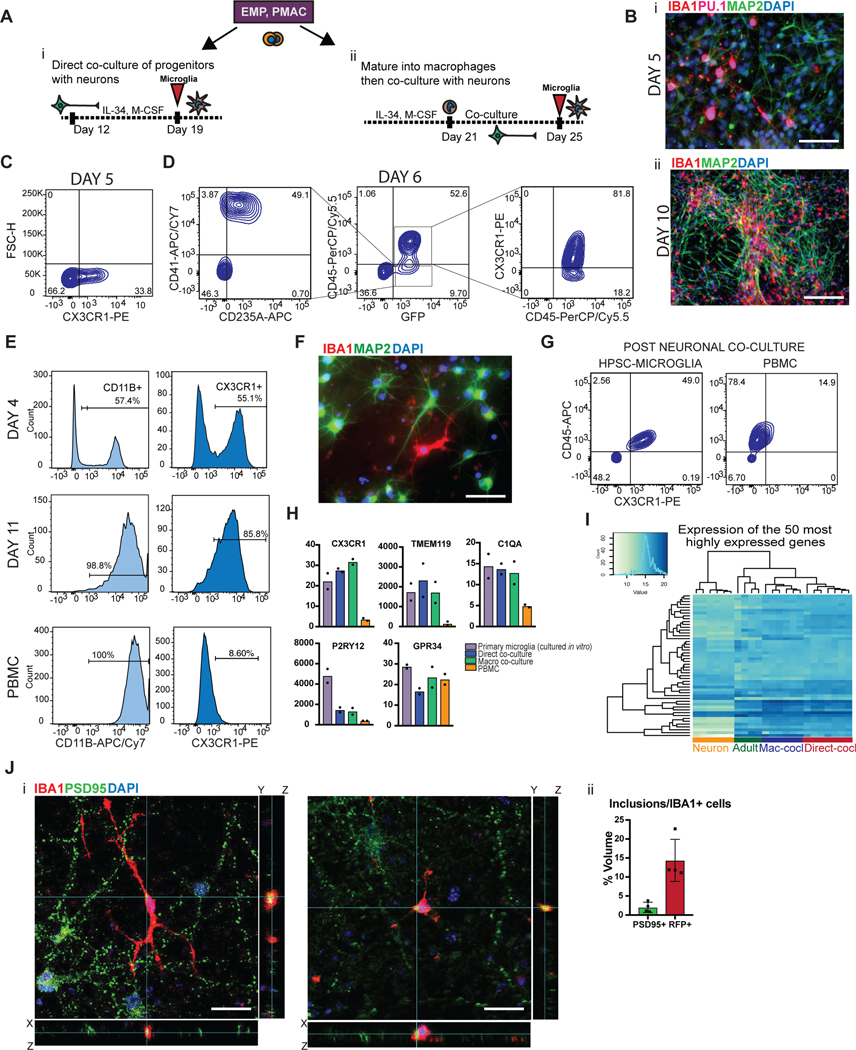
Figure 3. Two different methods to derive hPSC-microglia from the PMAC stage that are molecularly and functionally similar to primary microglia.
A) Schematic for two methods to derive microglia from PMACS. i) Day 10 microglial progenitors are co-cultured with postmitotic hPSC-derived neurons for 1 week in the presence of IL-34 and M-CSF. ii) Day 10 microglial progenitors are matured into primitive macrophages with IL-34 and M-CSF, then co-cultured with hPSC-derived neurons for 4 days. B)i. IF shows ramified IBA1+PU.1+ cells after day 5 of direct co-culture of day 10 progenitors with postmitotic hPSC-derived cortical neurons. Scale bar = 50 μM. ii. IF shows IBA1+ cells distributed evenly throughout the neuronal culture by day 10 of co-culture. Scale bar = 200uM. C) FACS analysis shows that over 30% of cells in co-culture at day 5 express CX3CR1+. D) FACS analysis shows that GFP+ day 10 progenitors co-cultured with hPSC-derived cortical neurons for 6 days are ~84% CD45+ indicating commitment to the microglial lineage. Of these over 80% are CX3CR1+, indicating maturation into early microglia. The ~15% GFP+ population that is CD45- is ~50% CD41+CD235A+ (primitive EMPs), and ~50% uncommitted. E) FACS analysis shows that maturing Day 10 progenitors in IL-34 and M-CSF without neurons yields a progressively pure population of primitive macrophages expressing CD11B (~99%) and CX3CR1 (>85%) by 11 days in culture. PBMCs matured in parallel express CD11B (100%) but not CX3CR1+ at day 11. F) IF shows ramified IBA1+ microglial-like cells after culturing primitive macrophages with hPSC-derived cortical neurons for 4 days. Scale bar = 50 μM. G) FACS analysis shows that co-cultured microglial cells maintain expression of CX3CR1 and have a lower expression of CD45 than PBMC-matured macrophages co-cultured with cortical neurons, which largely do not express CX3CR1 and have higher CD45 expression. H) qPCR of a panel of microglial-specific genes shows that hPSC-microglia generated from either method express these genes at similar levels to human primary microglial cDNA (Celprogen, commercially available), whereas PBMC-derived macrophages do not. Fold change is over day 10 progenitors for CX3CR1, TMEM119, C1QA, GPR34, and over hESCs for P2RY12. n=2 technical replicates of a representative qPCR. I) Bulk RNA-sequencing of microglia derived from 2 different methods (Mac-cocl = method ii, matured alone then co-cultured, Direct-cocl = method i, direct co-culture) show similarity to each other and to acutely isolated adult primary microglia from postmortem samples. hPSC-derived neurons sorted from co-culture grouped separately. n=3 samples per group. J) i) Confocal imaging shows microglia co-cultured with d70 hPSC-derived cortical neurons for 30 days contain inclusions of postsynaptic proteins (PSD95). Scale bar = 50 μM. ii) Quantification of inclusions show that inclusions containing general neuronal matter (tagged with RFP) are greater in volume than inclusions containing postsynaptic protein (PSD95). n=4 fields. Error bars = SD, center = mean.