Abstract
Objective
Vagus nerve stimulator (VNS) implantation is an established therapy for pharmacoresistant epilepsy that is not amenable to curative epilepsy surgery. Historically, VNS implantation has been performed by neurosurgeons, but otolaryngologist involvement is increasingly common. In this retrospective study, we aimed to evaluate the efficacy and safety of VNS implantation in children and adolescents from the otolaryngologists’ perspective.
Methods
This study included children and adolescents who had undergone VNS implantation at the study center between 2014 and 2018. Patient files were analyzed with regards to the durations of device implantation and hospitalization, postoperative complications, and clinical outcome, including seizure frequency, clinical global impression of improvement (CGI-I) score, and quality of life (QoL).
Results
A total of 73 children underwent VNS surgery. The median age at implantation was 9.3 ± 4.6 years, and median epilepsy duration before VNS surgery was 6 ± 4 years. Lennox–Gastaut syndrome was the most common syndrome diagnosis (62.3%), and structural abnormalities (49.3%) the most frequent etiology. Operation times ranged from 30 to 200 min, and median postoperative hospitalization length was 2 ± 0.9 days. No complications occurred, except for four revisions and two explantations due to local infections (2.7%). Among our patients, 76.7% were responders (≥ 50% reduction in seizure frequency), 72.1% showed improved CGI-I scores, and 18.6–60.5% exhibited considerable improvements in the QoL categories energy, emotional health, and cognitive functions.
Conclusion
Our results indicate that VNS implantation is a highly effective and safe treatment option for children and adolescents with AED-refractory epilepsies who are not candidates for curative epilepsy surgery.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00405-021-06943-x.
Keywords: Vagus nerve stimulator (VNS) surgery, Children, Epilepsy, Outcome, Perioperative management
Introduction
Epilepsy affects approximately 50 million people worldwide [1], one-fifth of whom are children under 15 years of age, who exhibit a wide-ranging clinical spectrum of this disease [2]. While most affected children are successfully treated with antiepileptic drugs (AEDs), 20–30% show pharmacoresistance, which is associated with high risks of intellectual disability, neuropsychiatric co-morbid disorders, increased mortality, and poor quality of life (QoL) [1, 3, 4]. Vagus nerve stimulation was approved for the treatment of AED-refractory epilepsy in the 1990s, and over 100,000 VNS devices have now been implanted worldwide. Vagus nerve stimulation is a well-established adjunctive therapy for epilepsy that is refractory to medical treatment but does not fulfil the criteria for respective/disconnective curative epilepsy surgery [5, 6]. Vagus nerve stimulation leads to persistent improvement of seizure control in ~ 50% of patients, with even better outcomes among children [4, 7–16].
Potential perioperative and postoperative complications of vagus nerve stimulation include intraoperative bradycardia and asystolia during lead impedance testing (0.01%), infections (3–8%), peritracheal hematoma, and nerve injury [17–26]. Later complications may be related to suboptimal vagal stimulation, recurrent laryngeal nerve co-stimulation, and/or mechanical stress—and can include delayed arrhythmias (bradycardia and asystolia), obstructive sleep apnea, and laryngopharyngeal dysfunction (dysphonia, cough, cervical pain, pharyngeal problems, and dyspnea). These complications occur in up to 66% of patients, but are often controllable by adjusting the stimulation parameters [6, 13, 17–26]. Drooling and hyperactivity have also occasionally been reported in children [14].
After primary VNS implantation, 4–16.8% of patients undergo revisions due to device malfunction, VNS therapy failure, intolerable side effects, or patient dissatisfaction [21–26]. The spectrum of these complications suggests that successful device implantation and positioning are critical. While VNS implantation has historically been performed by neurosurgeons [27], otolaryngologists are increasingly involved in this procedure due to their expertise regarding the surgical anatomy of the neck, and their far-reaching experience in the implantation of neuromodulation devices, such as the cochlear implants, and devices for hypoglossal nerve stimulation or and baroreflex modulation.
In the present study, we retrospectively evaluated various aspects of VNS therapy, to weigh the risks of device implantation against the possible benefits for seizure control and QoL.
Materials and methods
We performed a retrospective study of all patients who had undergone a VNS implantation or a secondary VNS-related surgery at the Department of Otorhinolaryngology, Head and Neck Surgery of the Medical University of Vienna between March 2014 and December 2018.
Data assessment
Data were obtained from the electronic epilepsy registry, which includes all patients who have undergone presurgical evaluation at the Department of Pediatrics and Adolescent Medicine since 2000. Patients’ records were retrospectively evaluated to retrieve sociodemographic data, epilepsy type, comorbidities, procedure, surgery length, immediate intraoperative and postoperative complications, duration and mode of postoperative care, vagus nerve stimulation-associated side effects, and AED treatment before VNS implantation and at last consultation.
Preoperative period
To determine candidacy for epilepsy surgery, all patients underwent a standard-of-care presurgical evaluation at the MUW pediatric epilepsy center, which included long-term video-EEG-monitoring; neurological, neuroophthalmological, neuropsychological, and neuropsychiatric investigations; and MRI. Indications for implantation were discussed and decided at the MUW pediatric epilepsy interdisciplinary seizure conference.
Surgical procedure
All VNS implantations were performed by the senior coauthors (W-D.B. and W.G.). The implantation procedure and technique was similar to that described by Reid et al. [28]. Supplementary Fig. S1 illustrates the most important steps of VNS surgery.
Postoperative period
Following VNS implantation, the parameter settings were titrated on an outpatient basis by the treating epileptologists at the MUW pediatric epilepsy center (A.D., G.G., R.D., and M.F.). The patients’ caregivers were asked to keep seizure diaries—including seizure frequency, severity, and times of occurrence—before and after VNS implantation. The baseline parental seizure count of the primary seizure type prior to VNS implantation was compared with that at last observation. Patients showing a seizure frequency reduction of ≥ 50% were defined as “responders”.
QoL was assessed based on available patient records, and on standardized telephone interviews with caregivers, including evaluation of energy/activity level, concentration, alertness, memory, verbal communication, mood, performance at school, and development of life skills. QoL was categorized as better or much better since VNS, unchanged since VNS, worse or much worse since VNS, or unknown.
The Clinical Global Impression–Improvement scale (CGI-I) was used to assess whether a patient’s illness had improved or worsened compared to baseline before implantation. CGI-I scores were stratified as “much improved or very much improved”, “minimally improved”, “no change”, or “minimally worse or very much worse”. Postoperative pain was assessed using a visual numerical rating scale (vNRS), where 0 indicated no pain and 10 indicated maximal pain. This scoring was possible in 27 (37%) of 73 children, and was not applicable in the remaining children due to intellectual disability and limited communication.
Statistical analysis
Statistical analyses were performed using SPSS version 26.0 software (IBM Corp. Armonk, NY, USA). Data are presented as median and mean ± standard deviation (SD) unless otherwise stated. Descriptive statistics were applied to evaluate the significance of demographic and clinical data, and were used for both subjective and objective quantitative measurable responses. The chi-squared test was used to compare nominal variables. We used the Pearson and Spearman correlations (correlation coefficient, r) to analyze linear relationships between metric and ranked variables, respectively. A P value of < 0.05 was considered statistically significant.
Results
Study cohort
Our analysis included 73 patients, comprising 32 girls (43.8%) and 41 boys (56.2%), with a median age of 9.3 ± 4.6 years (1.9–20.1 years). All children suffered from AED-resistant epilepsy. The median disease duration before VNS implantation was 6 ± 4 years (0.4–16.7 years). Patients had received a median of 6 ± 3.2 different AEDs (2–15 AEDs). Twelve children (16.4%) followed a ketogenic diet (KD). Prior to VNS implantation, six children (8.2%) had undergone resective epilepsy surgeries with unfavorable outcomes, including temporal lobe resection (n = 2), frontal lobe resection (n = 2), callosotomy (n = 1), and hemispherotomy (n = 1).
The most common epilepsy syndrome was Lennox–Gastaut syndrome (n = 44; 60.3%), followed by West syndrome (WS) (n = 6; 8.2%), epileptic encephalopathy with continuous spike and wave during slow wave sleep (CWSWS) (n = 6; 8.2%), Dravet syndrome (DS) (n = 5; 6.8%), epilepsy with myoclonic-atonic seizures (MAE) (n = 5; 6.8%), epilepsy with myoclonic absences (n = 4; 5.4%), combined generalized and focal epilepsy (n = 2; 2.8%), and focal epilepsy (n = 1; 1.4%) (Table 1). The most common etiology was structural (n = 36; 49.3%), followed by combined genetic and structural (n = 16, 21.9%), and genetic (n = 11, 15.1%). MRI revealed epileptogenic lesions in 54 cases (74%). Table 1 presents the types of epilepsy and seizures, and electroencephalography (EEG) characteristics.
Table 1.
Epilepsy and seizure characteristics of all children (n = 73)
| No. of patients (%) | ||
|---|---|---|
| Syndromes |
Lennox–Gastaut syndrome West syndrome CSWS epilepsy Dravet syndrome Epilepsy with myoclonic-atonic seizures Epilepsy with myoclonic absences Combined generalized and focal epilepsy Focal epilepsy |
44 (60.3) 6 (8.2) 6 (8.2) 5 (6.8) 5 (6.8) 4 (5.4) 2 (2.8) 1 (1.4) |
| Seizure types |
Focal onset Generalized onset Unknown onset |
38 (52.1) 34 (46.6) 1 (1.4) |
| Epilepsy types |
Focal Generalized Combined: generalized & focal Unknown |
38 (52.1) 22 (30.1) 12 (16.4) 1 (1.4) |
| Etiology |
Structural Structural and genetical Unknown Genetic Infectious and structural Metabolic |
36 (49.3) 16 (21.9) 11 (15.1) 6 (8.2) 3 (4.1) 1 (1.4) |
| EEG characteristics | ||
| Brain function disorder |
None Diffuse Focal Severe Missing |
29 (39.7) 26 (35.6) 15 (20.5) 2 (2.7) 1 (1.4) |
| Cerebral excitability |
None Focal Multifocal Generalized Missing |
5 (6.8) 12 (16.4) 41 (56.2) 13 (17.8) 2 (2.7) |
| EEG pathology grade |
I II III Missing |
1 (1.4) 1 (1.4) 70 (95.9) 1 (1.4) |
CSWS epileptic encephalopathy with continuous spike and wave during sleep, EEG electroencephalography
Surgical procedure and perioperative period
A total of 54 primary VNS implantations were performed using Cyberonics 106 (n = 36), Cyberonics 103 (n = 9), Cyberonics 304 (n = 6), Sentiva 1000 (n = 2), and Cyberonics 303 (n = 1) devices, with no reported device failure. Of these 54 cases, 4 required generator replacement (all Cyberonics 106) during the observation period. Additionally, 15 generators that were implanted at other institutions between 2007 and 2014 had to be exchanged due to end of battery service, and were replaced by Cyberonics 103 (n = 4) or Cyberonics 106 generators (n = 11). Overall, the generators’ running time was 5.1 ± 1.6 years (2.5–7.7 years). Four complete system changes were performed due to electrode fractures. The primary implantations had been performed at other institutions between 2001 and 2013, with a median running time of 7.7 ± 3.4 years (4.9–14.1 years).
The median duration of surgery was 100 ± 17.1 min (70–165 min) for primary VNS implantation, 45 ± 12.1 min (30–60 min) for generator replacements, and 175.5 ± 54.3 min (85–200 min) for complete system change. The median impedance, measured during surgery, was 1600 ± 387.9Ω (980–3485 Ω). After VNS implantation, four cases (5.2%) required intensified observation at an intermediate care unit due to patients’ underlying diseases. In the remaining 73 (94.8%) interventions, the patients were transferred to the normal inpatient ward after a regular observation time in the recovery room. The median postoperative inpatient stay was 2 ± 0.9 days (0–5 days) with no significant differences between primary implantation, generator replacement, and complete system change.
Postoperative pain was managed using paracetamol alone (n = 29) or combined with metamizole or other non-steroidal anti-inflammatory drugs (NSAIDs) (n = 21). According to vNRS, the average pain level was 1 ± 0.9 (out of 10) (0–3). Perioperative antibiotic therapy was administered either as single-shot treatment or as perioperative and postoperative therapy over 9 ± 1.3 days (2–12 days). An amino-penicillin was used in 93.2% of patients.
Complications and side effects
No severe complications (e.g., bleedings or asystolia) occurred during surgery. Dysphonia was noted in three cases (3.9%). One 2-year-old child suffering from WS exhibited a single self-limiting episode of postoperative bradycardia, which did not seem to be related to VNS implantation since the stimulation parameters of the VNS device had not been changed, and the bradycardia was self-limiting.
Two patients (2.7%) suffered an infection-associated incompatibility reaction against the implant, and were thus explanted and not re-implanted. In both children, the infection was localized at the generator area, and was first observed a month after surgery due to a local swelling fistulation and accompanying secretion. Three children died due to their underlying diseases.
Seizure frequency and improvement after VNS implantation
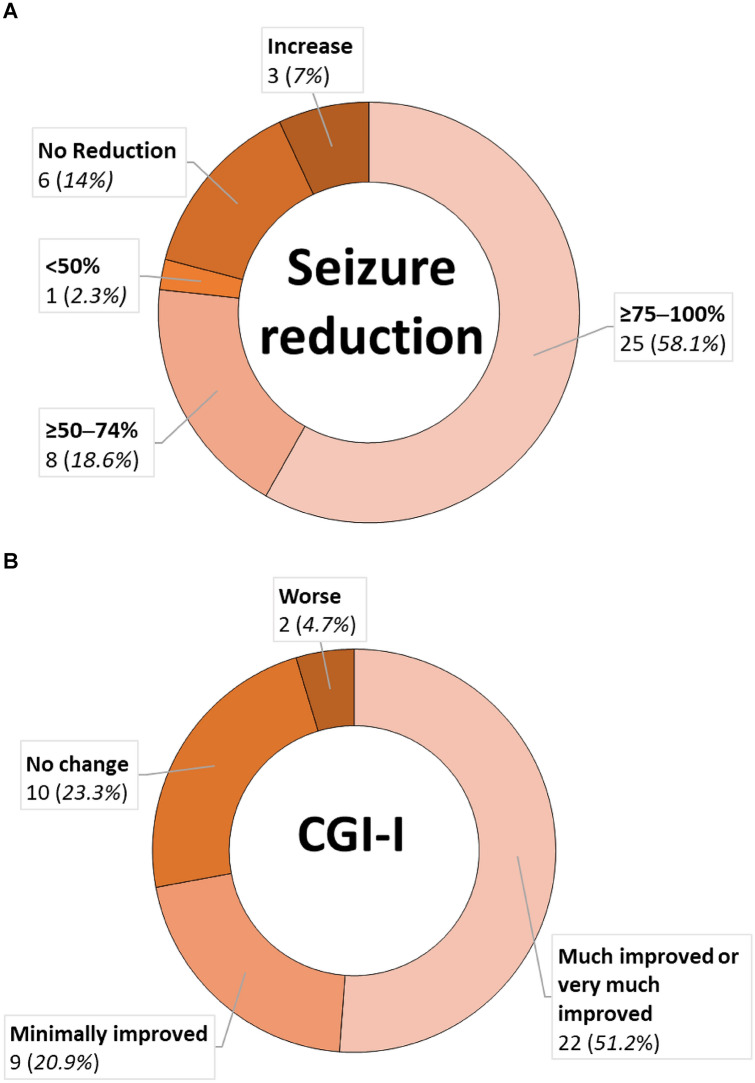
Analyses for outcome assessment included only the patients with primary VNS implantation (n = 54). Six children were lost to follow-up, two required explantation, and three were deceased. Therefore, our analysis included follow-up data from 43 patients. The mean follow-up time was 3 ± 1.3 years (1–5.9 years). Among our patients, 76.7% were responders (n = 33), including 25 patients with a seizure frequency reduction of ≥ 75% after VNS implantation. No patient became seizure-free. One patient (2.3%) exhibited a frequency decrease of < 50%, six (14%) showed no improvement, and three (7%) showed an increased seizure frequency (Fig. 1A).
Fig. 1.
Reduction of seizure frequency A and results of the clinical global impression of improvement (CGI-I) assessment B after vagus nerve stimulation device implantation
Etiology was a significant factor associated with therapy response. Patients with exclusively structural, unknown, or genetic etiologies showed a significantly higher therapy response than the remaining patients (P = 0.048). The various seizure syndromes were not associated with seizure outcome (P = 0.148). Seizure outcome was significantly correlated with age at seizure onset (P = 0.025; r = − 0.363), with older age at onset associated with better response and seizure frequency reduction. Previous unsuccessful resective epilepsy surgery (P = 0.032) was also significantly associated with unfavorable seizure outcome in terms of seizure frequency. Seizure outcome was not correlated with sex, number of previously used AEDs, age at time of VNS implantation, duration of epilepsy prior to VNS surgery, or epilepsy-specific factors (e.g., EEG characteristics and types of seizure and epilepsy) (Table 2).
Table 2.
Overview of factors correlating with objective and subjective therapy response after vagus nerve stimulator (VNS) implantation
| Responder | CGI-I | |||
|---|---|---|---|---|
| P | r | P | r | |
| Age at seizure onset | 0.025 | − 0.363a | 0.237 | − 0.215b |
| Age at VNS surgery | 0.491 | 0.108a | 0.001 | 0.471 b |
| Sex | 0.769 | –c | 0.146 | –c |
| Previous curative epilepsy surgeries | 0.032 | –c | 0.228 | –c |
| Duration of epilepsy before VNS | 0.483 | − 0.121a | 0.023 | 0.407b |
| Number of AEDs before VNS | 0.052 | 0.772a | 0.261 | 0.182b |
| Epilepsy syndromes | 0.147 | –c | 0.330 | –c |
| Epilepsy types | 0.073 | –c | 0.332 | –c |
| Seizure types | 0.279 | –c | 0.559 | –c |
| Etiology | 0.048 | –c | 0.013 | –c |
| QoL | ||||
| Alertness | 0.686 | − 0.089a | 0.003 | 0.443b |
| Concentration | 0.637 | − 0.104a | < 0.0001 | 0.569b |
| Energy | 0.611 | 0.112a | 0.001 | 0.486b |
| Memory | 0.978 | 0.004a | 0.986 | 0.003b |
| Mood | 0.984 | 0.006a | < 0.0001 | 0.576b |
| Verbal communication | 0.399 | − 0.185a | 0.029 | 0.334b |
| Progress at schoolwork | 0.683 | 0.090a | 0.037 | 0.319b |
| Development of life skills | 0.236 | 0.257a | 0.320 | 0.155b |
QoL quality of life, AED antiepileptic drugs, P P value, r = correlation coefficient
aPearson correlation
bSpearman
cChi-squared test correlation
QoL and global impression of improvement after VNS implantation
Alertness increased in 60.5% (26/43) of the patients, and was the parameter that was most commonly improved by VNS therapy. We also found considerable amelioration of concentration (39.5%; 17/43), energy (41.9%; 18/43), mood (41.9%; 18/43), verbal communication (34.9%, 15/43), progress in schoolwork (39.5%; 17/43), memory (20.9%; 9/43), and development of life skills (18.6%; 8/43). After implantation, four patients (9.3%) exhibited reduced energy levels, and two exhibited aggravation of various aspects of QoL (Table 3).
Table 3.
Quality of life (QoL) assessment after vagus nerve stimulator (VNS) implantation
| No. of patients (%) | |
|---|---|
| Alertness | |
| Better or much better than before VNS | 26 (60.5%) |
| Unchanged since VNS | 14 (32.6%) |
| Worse or much worse than before VNS | 1 (2.3%) |
| Not known | 2 (4.7%) |
| Concentration | |
| Better or much better than before VNS | 17 (39.5%) |
| Unchanged since VNS | 22 (51.2%) |
| Worse or much worse than before VNS | 1 (2.3%) |
| Not known | 3 (7%) |
| Energy | |
| Better or much better than before VNS | 18 (41.9%) |
| Unchanged since VNS | 19 (44.2%) |
| Worse or much worse than before VNS | 4 (9.3%) |
| Not known | 2 (4.7%) |
| Memory | |
| Better or much better than before VNS | 9 (20.9%) |
| Unchanged since VNS | 15 (34.9%) |
| Worse or much worse than before VNS | 1 (2.3%) |
| Not known | 18 (41.9%) |
| Mood | |
| Better or much better than before VNS | 18 (41.9%) |
| Unchanged since VNS | 17 (39.5%) |
| Worse or much worse than before VNS | 2 (4.7%) |
| Not known | 6 (14%) |
| Verbal communication | |
| Better or much better than before VNS | 15 (34.9%) |
| Unchanged since VNS | 19 (44.1%) |
| Worse or much worse than before VNS | 2 (4.7%) |
| Not known | 7 (16.3%) |
| Progress with schoolwork | |
| Better or much better than before VNS | 17 (39.5%) |
| Unchanged since VNS | 15 (34.9%) |
| Worse or much worse than before VNS | 0 |
| Not known | 11 (25.6%) |
| Development of life skills | |
| Better or much better than before VNS | 8 (18.6%) |
| Unchanged since VNS | 23 (53.5%) |
| Worse or much worse than before VNS | 1 (2.3%) |
| Not known | 11 (25.6%) |
The CGI-I scores, based on the parents’ assessment of the patients’ overall condition, were significantly correlated with all QoL parameters, except memory and development of life skills (Table 2). According to the CGI-I scale, 51.2% (22/43) of the patients were “much improved” or “very much improved”, 20.9% (9/43) were “minimally improved” (Fig. 1B), and 28% exhibited no change or even a deterioration of the overall condition (4.7%). A positive CGI-I was significantly correlated with age at VNS surgery (P = 0.001; r = 0.471) and duration of epilepsy prior to VNS implantation (P = 0.023; r = 0.407) (Table 2). Children showing the highest benefit had a mean age of 8.9 years (SD: ± 4.3 years) and a mean epilepsy duration of 5.6 years (SD: ± 3.8 years), while children showing low/no improvement or deterioration of QoL had a mean age of 10.9 years (SD: ± 5.1 years) and a mean epilepsy duration of 8.5 years (SD: ± 3.7 years) (P = 0.022).
Etiology was also significantly associated with CGI-I improvement or deterioration. Seizure forms with a structural etiology exhibited significant improvement of CGI-I after VNS implantation (P = 0.029). Age at seizure onset, sex, previous anticonvulsive surgery or AED treatment, and other seizure characteristics were not associated with QoL parameters (Table 2). Finally, we examined the relationship between treatment response and QoL, and correlations between data from seizure responders and QoL responders. Seizure responders and non-responders did not differ in either CGI-I (P = 0.839, r = 0.037) or epilepsy-specific QoL after VNS implantation (Table 2).
Discussion
In this retrospective study, we evaluated the short- and long-term effects and risks associated with VNS implantation in children and adolescents with pharmaco-resistant epilepsies. Little has been published about the increasing involvement of otolaryngologists in this surgical intervention and the immediate postoperative management. In the present study, surgical and perioperative care were provided by otolaryngologists, while neuropediatricians were responsible for the indication and the preoperative and postoperative care. This requires intense interdisciplinary communication and cooperation, especially during the postoperative phase when the first adjustments of VNS parameters are required.
Among our patients, the mean surgery duration varied from 30 to 200 min, depending on whether all components were implanted or only single components of the VNS had to be replaced which is in accordance with previously reported operation times [29, 30]. Our perisurgically measured mean impedance of 1580.9 Ω is also within previously reported limits [31]. Interestingly, the children did not consider the procedure itself to be very painful, with an average reported pain level of 1/10. Correspondingly, perioperative administration of paracetamol and/or NSAIDs was sufficient.
Concerns have been raised about patient safety due to reports of severe perioperative and postoperative complications, including major bleeding, cardiac arrhythmias, asystolia leading to cardiopulmonary resuscitation (CPR), and vocal cord paralysis [6, 13]. None of these severe events were reported in our patient group. Of the 73 children, 2 (2.8%) developed an incompatibility reaction against the implant, resulting in local infection of soft tissue, and requiring several revisions and ultimately explantation. These observations are in alignment with previously reported infection rates of ~ 5% [32–34]. The rarity of adverse effects was reflected by the short average hospital stay of only 2 days for all VNS surgery types. Most children were postoperatively transferred to the normal ward, with only a few cases requiring transition to an intermediate or intensive care unit. No persistent vocal cord dysmotility or paresis occurred. Transient stimulation-related dysphonia was observed in 3.9% of cases, and disappeared when parameter settings were changed (i.e., pulse width and strength).
With regards to the efficacy of vagus nerve stimulation for seizure reduction, 76.7% of our patients were “responders”, showing a seizure frequency reduction of ≥ 50%. Only 21% experienced no response or aggravation of seizure frequency. Previous reports show less favorable outcomes after VNS surgery [4, 35, 36]. Several aspects may have contributed to the success of VNS therapy in our patients. First, our pediatric collective was relatively young at VNS implantation, with a mean age of 10.4 years, and younger age was significantly correlated with better seizure outcomes. Several reports from other centers also show that VNS therapy is more efficient in children than adults [4, 35]. Additionally, the involved neuropediatricians had long-standing expertise in patient selection for VNS implantation, and subsequent medical assistance and treatment. Seizure control is a challenging and dynamic process [4]. Seizure frequency tends to increase with any infectious disease, and children may readily deteriorate. Moreover, 93% of our patients were still receiving AEDs at last follow-up, and it is difficult to disentangle the possible interactions between AED and vagus nerve stimulation with regards to seizure reduction.
Importantly, epilepsy treatment involves more than seizure counting. Reductions in seizure intensity and duration can have positive effects on the day-to-day lives of children with refractory epilepsy. This may explain the absence of a clear relationship between VNS-induced reduction in seizure frequency and improved QoL, as observed in the present study and in previous reports [16, 37].
In terms of QoL, we observed significant improvements in the parent-rated scores for energy and emotional health compared to preoperative assessments. Vagus nerve stimulation increases extracellular norepinephrine and serotonin availability in mood-regulating areas, e.g., the limbic system and prefrontal cortex. The mode of action resembles that of antidepressant medication, based on serotonin and norepinephrine reuptake inhibition [38, 39]. Positron emission tomography and single-photon emission computed tomography imaging studies in depressed patients demonstrate that vagus nerve stimulation changes the activity in the limbic system and prefrontal cortex [40, 41]. Regarding cognitive functions, we tested alertness, concentration, memory, and performance at schoolwork, and most patients showed considerable improvement after vagus nerve stimulation compared to baseline. Increasing evidence suggests that the stimulation may impact neuromodulatory systems, such as the locus coeruleus and the cholinergic systems, which modulate cognitive functions [42, 43]. Vagus nerve stimulation impacts different brain areas and neurotransmitter systems, with variation depending on the impulse frequency and magnitude [43].
Previously published work has mostly focused on single aspects of VNS surgery, therefore, our overall aim was to provide a comprehensive overview of the practical and holistic components of VNS surgery as seen from the point of head & neck. We describe the surgical process, consider the pre- and postoperative management including our favorable outcome. We also show the importance of efficient and patient centered multidisciplinary management, which is of upmost importance to ensure optimal patient selection and postoperative care from the experience of head & neck surgery. From the neuropediatric point of view, we believe that the presentation and comparison of both, objective and subjective outcome data after VNS surgery in a pediatric cohort, is of specific high value. To the best of our knowledge, this work up of VNS surgery is a quite unique approach. The major drawback of this study is its retrospective nature and an inherent bias due to the selection of information. Moreover, the heterogeneity of our patients, with a broad spectrum of different etiologies and epilepsy syndromes, limits the significance of predicting improvement after VNS implantation.
Conclusion
Children and adolescents suffering from AED-refractory epilepsy greatly benefit from VNS therapy, substantiating the need to avoid delays in patient selection and VNS treatment. Otolaryngologist should be encouraged to apply their specific expertise in neck surgery towards the implantation of VNS devices, as it is an established surgical procedure that provides highly satisfactory outcomes in terms of safety and efficacy.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Meeting information
Preliminary data from this work were presented at the 63rd Annual Meeting of the Austrian Society of Otorhinolaryngology, Head and Neck Surgery, 11–15. September 2019, Salzburg, Austria.
Author contributions
SG, study design, data acquisition, analysis and interpretation, manuscript drafting, final approval, and accountability for all aspects of the work; SJ, study design, data analysis and interpretation, manuscript revising, final approval, and accountability for all aspects of the work; AD, study design, data acquisition and interpretation, manuscript revising, final approval, and accountability for all aspects of the work; RD, data acquisition, manuscript revising, final approval, and accountability for all aspects of the work; GG, data acquisition and interpretation, manuscript revising, final approval, and accountability for all aspects of the work; KE, study design, data analysis and interpretation, manuscript revising, final approval, and accountability for all aspects of the work; MG, study design, data acquisition and interpretation, manuscript revising, final approval, and accountability for all aspects of the work; WG, data acquisition, manuscript revising, final approval, and accountability for all aspects of the work; MF, study design, data analysis and interpretation, manuscript drafting, final approval, and accountability for all aspects of the work; EV, study design, data analysis and interpretation, manuscript drafting, final approval, and accountability for all aspects of the work; WDB, study design, data analysis and interpretation, manuscript drafting, final approval, and accountability for all aspects of the work.
Funding
Open access funding provided by Medical University of Vienna.
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
All authors declare that they have no competing interests.
Ethical approval
Ethical approval was obtained from the Ethics Committee of the Medical University of Vienna (EK 1530/2019, with annual extensions to date).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
M. Feucht, Email: martha.feucht@meduniwien.ac.at
E. Vyskocil, Email: erich.vyskocil@meduniwien.ac.at
References
- 1.Kwan P, Brodie MJ. Early identification of refractory epilepsy. N Engl J Med. 2000;342:314–319. doi: 10.1056/NEJM200002033420503. [DOI] [PubMed] [Google Scholar]
- 2.Guerrini R. Epilepsy in children. Lancet. 2006;367:499–524. doi: 10.1016/S0140-6736(06)68182-8. [DOI] [PubMed] [Google Scholar]
- 3.Berg AT, Zelko FA, Levy SR, et al. Age at onset of epilepsy, pharmacoresistance, and cognitive outcomes: a prospective cohort study. Neurology. 2012;79:1384–1391. doi: 10.1212/WNL.0b013e31826c1b55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Orosz I, McCormick D, Zamponi N, et al. Vagus nerve stimulation for drug-resistant epilepsy: a European long-term study up to 24 months in 347 children. Epilepsia. 2014;55:1576–1584. doi: 10.1111/epi.12762. [DOI] [PubMed] [Google Scholar]
- 5.Landy HJ, Ramsay RE, Slater J, et al. Vagus nerve stimulation for complex partial seizures: surgical technique, safety, and efficacy. J Neurosurg. 1993;78:26–31. doi: 10.3171/jns.1993.78.1.0026. [DOI] [PubMed] [Google Scholar]
- 6.Ramsay RE, Uthman BM, Augustinsson LE, et al. Vagus nerve stimulation for treatment of partial seizures: 2. Safety, side effects, and tolerability. First International Vagus Nerve Stimulation Study Group. Epilepsia. 1994;35:627–636. doi: 10.1111/j.1528-1157.1994.tb02483.x. [DOI] [PubMed] [Google Scholar]
- 7.Elliott RE, Morsi A, Kalhorn SP, et al. Vagus nerve stimulation in 436 consecutive patients with treatment-resistant epilepsy: long-term outcomes and predictors of response. Epilepsy Behav. 2011;20:57–63. doi: 10.1016/j.yebeh.2010.10.017. [DOI] [PubMed] [Google Scholar]
- 8.Morris GL, 3rd, Mueller WM. Long-term treatment with vagus nerve stimulation in patients with refractory epilepsy. The Vagus Nerve Stimulation Study Group E01–E05. Neurology. 1999;53:1731–1735. doi: 10.1212/WNL.53.8.1731. [DOI] [PubMed] [Google Scholar]
- 9.Cukiert ACC, Burattini JA, Lima AM, et al. A prospective long-term study on the outcome after vagus nerve stimulation at maximally tolerated current intensity in a cohort of children with refractory secondary generalized epilepsy. Neuromodulation. 2012;16:551–556. doi: 10.1111/j.1525-1403.2012.00522.x. [DOI] [PubMed] [Google Scholar]
- 10.Morris GL, 3rd, Gloss D, Buchhalter J, et al. Evidence-based guideline update: vagus nerve stimulation for the treatment of epilepsy: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;81:1453–1459. doi: 10.1212/WNL.0b013e3182a393d1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hauptman JS, Mathern GW. Vagal nerve stimulation for pharmacoresistant epilepsy in children. Surg Neurol Int. 2012;3(Suppl 4):S269–S274. doi: 10.4103/2152-7806.103017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arya R, Greiner HM, Lewis A, et al. Vagus nerve stimulation for medically refractory absence epilepsy. Seizure. 2013;22:267–270. doi: 10.1016/j.seizure.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 13.Thompson EM, Wozniak SE, Roberts CM, et al. Vagus nerve stimulation for partial and generalized epilepsy from infancy to adolescence. J Neurosurg Pediatr. 2012;10:200–205. doi: 10.3171/2012.5.PEDS11489. [DOI] [PubMed] [Google Scholar]
- 14.Helmers SL, Duh MS, Guerin A, et al. Clinical outcomes, quality of life, and costs associated with implantation of vagus nerve stimulation therapy in pediatric patients with drug-resistant epilepsy. Eur J Paediatr Neurol. 2012;16:449–458. doi: 10.1016/j.ejpn.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 15.Alexopoulos AV, Kotagal P, Loddenkemper T, et al. Long-term results with vagus nerve stimulation in children with pharmacoresistant epilepsy. Seizure. 2006;15:491–503. doi: 10.1016/j.seizure.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 16.Patwardhan RV, Stong B, Bebin EM, et al. Efficacy of vagal nerve stimulation in children with medically refractory epilepsy. Neurosurgery. 2000;47:1353–1357. doi: 10.1097/00006123-200012000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Zalvan C, Sulica L, Wolf S, et al. Laryngopharyngeal dysfunction from the implant vagal nerve stimulator. Laryngoscope. 2003;113:221–225. doi: 10.1097/00005537-200302000-00005. [DOI] [PubMed] [Google Scholar]
- 18.Fahy BG. Intraoperative and perioperative complications with a vagus nerve stimulation device. J Clin Anesth. 2010;22:213–222. doi: 10.1016/j.jclinane.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Rychlicki F, Zamponi N, Cesaroni E, et al. Complications of vagal nerve stimulation for epilepsy in children. Neurosurg Rev. 2006;29:103–107. doi: 10.1007/s10143-005-0005-5. [DOI] [PubMed] [Google Scholar]
- 20.Spuck S, Tronnier V, Orosz I, et al. Operative and technical complications of vagus nerve stimulator implantation. Neurosurgery. 2010;67:489–494. doi: 10.1227/NEU.0b013e3181f88867. [DOI] [PubMed] [Google Scholar]
- 21.Kahlow H, Olivecrona M. Complications of vagal nerve stimulation for drug-resistant epilepsy: a single center longitudinal study of 143 patients. Seizure. 2013;22:827–833. doi: 10.1016/j.seizure.2013.06.011. [DOI] [PubMed] [Google Scholar]
- 22.Couch JD, Gilman AM, Doyle WK. Long-term expectations of vagus nerve stimulation: a look at battery replacement and revision surgery. Neurosurgery. 2016;78:42–46. doi: 10.1227/NEU.0000000000000985. [DOI] [PubMed] [Google Scholar]
- 23.Revesz D, Rydenhag B, Ben-Menachem E. Complications and safety of vagus nerve stimulation: 25 years of experience at a single center. J Neurosurg Pediatr. 2016;18:97–104. doi: 10.3171/2016.1.PEDS15534. [DOI] [PubMed] [Google Scholar]
- 24.Tran Y, Shah AK, Mittal S. Lead breakage and vocal cord paralysis following blunt neck trauma in a patient with vagal nerve stimulator. J Neurol Sci. 2011;304:132–135. doi: 10.1016/j.jns.2011.02.022. [DOI] [PubMed] [Google Scholar]
- 25.Kalkanis JG, Krishna P, Espinosa JA, et al. Self-inflicted vocal cord paralysis in patients with vagus nerve stimulators. Report of two cases J Neurosurg. 2002;96:949–951. doi: 10.3171/jns.2002.96.5.0949. [DOI] [PubMed] [Google Scholar]
- 26.Clark A, Kupermann RA, Auguste KI, et al. Intractable episodic bradycardia resulting from progressive lead traction in an epileptic child with a vagus nerve stimulator: a delayed complication. J Neurosurg Pediatr. 2012;9:389–393. doi: 10.3171/2011.12.PEDS11124. [DOI] [PubMed] [Google Scholar]
- 27.Ghanem T, Early SV. Vagal nerve stimulator implantation: an otolaryngologist's perspective. Otolaryngol Head Neck Surg. 2006;135:46–51. doi: 10.1016/j.otohns.2006.02.037. [DOI] [PubMed] [Google Scholar]
- 28.Reid SA. Surgical technique for implantation of the neurocybernetic prosthesis. Epilepsia. 1990;31(Suppl 2):S38–S39. doi: 10.1111/j.1528-1157.1990.tb05847.x. [DOI] [PubMed] [Google Scholar]
- 29.Kirse DJ, Werle AH, Murphy JV, et al. Vagus nerve stimulator implantation in children. Arch Otolaryngol Head Neck Surg. 2002;128:1263–1268. doi: 10.1001/archotol.128.11.1263. [DOI] [PubMed] [Google Scholar]
- 30.Al Omari AI, Alzoubi FQ, Alsalem MM, et al. The vagal nerve stimulation outcome, and laryngeal effect: Otolaryngologists roles and perspective. Am J Otolaryngol. 2017;38:408–413. doi: 10.1016/j.amjoto.2017.03.011. [DOI] [PubMed] [Google Scholar]
- 31.Giordano F, Zicca A, Barba C, et al. Vagus nerve stimulation: surgical technique of implantation and revision and related morbidity. Epilepsia. 2017;58(Suppl 1):85–90. doi: 10.1111/epi.13678. [DOI] [PubMed] [Google Scholar]
- 32.Horowitz G, Amit M, Fried I, et al. Vagal nerve stimulation for refractory epilepsy: the surgical procedure and complications in 100 implantations by a single medical center. Eur Arch Otorhinolaryngol. 2013;270:355–358. doi: 10.1007/s00405-012-2118-0. [DOI] [PubMed] [Google Scholar]
- 33.Air EL, Ghomri YM, Tyagi R, et al. Management of vagal nerve stimulator infections: do they need to be removed? J Neurosurg Pediatr. 2009;3:73–78. doi: 10.3171/2008.10.PEDS08294. [DOI] [PubMed] [Google Scholar]
- 34.Galbarriatu L, Pomposo I, Aurrecoechea J, et al. Vagus nerve stimulation therapy for treatment-resistant epilepsy: a 15-year experience at a single institution. Clin Neurol Neurosurg. 2015;137:89–93. doi: 10.1016/j.clineuro.2015.06.023. [DOI] [PubMed] [Google Scholar]
- 35.Fan HC, Hsu TR, Chang KP, et al. Vagus nerve stimulation for 6- to 12-year-old children with refractory epilepsy: Impact on seizure frequency and parenting stress index. Epilepsy Behav. 2018;83:119–123. doi: 10.1016/j.yebeh.2017.12.009. [DOI] [PubMed] [Google Scholar]
- 36.Gurbani S, Chayasirisobhon S, Cahan L, et al. Neuromodulation therapy with vagus nerve stimulation for intractable epilepsy: a 2-year efficacy analysis study in patients under 12 years of age. Epilepsy Res Treat. 2016;2016:9709056. doi: 10.1155/2016/9709056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sherman EM, Connolly MB, Slick DJ, et al. Quality of life and seizure outcome after vagus nerve stimulation in children with intractable epilepsy. J Child Neurol. 2008;23:991–998. doi: 10.1177/0883073808315417. [DOI] [PubMed] [Google Scholar]
- 38.Raedt R, Clinckers R, Mollet L, et al. Increased hippocampal noradrenaline is a biomarker for efficacy of vagus nerve stimulation in a limbic seizure model. J Neurochem. 2011;117:461–469. doi: 10.1111/j.1471-4159.2011.07214.x. [DOI] [PubMed] [Google Scholar]
- 39.Manta S, El Mansari M, Debonnel G, et al. Electrophysiological and neurochemical effects of long-term vagus nerve stimulation on the rat monoaminergic systems. Int J Neuropsychopharmacol. 2013;16:459–470. doi: 10.1017/S1461145712000387. [DOI] [PubMed] [Google Scholar]
- 40.Conway CR, Sheline YI, Chibnall JT, et al. Brain blood-flow change with acute vagus nerve stimulation in treatment-refractory major depressive disorder. Brain Stimul. 2012;5:163–171. doi: 10.1016/j.brs.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kosel M, Brockmann H, Frick C, et al. Chronic vagus nerve stimulation for treatment-resistant depression increases regional cerebral blood flow in the dorsolateral prefrontal cortex. Psychiatry Res. 2011;191:153–159. doi: 10.1016/j.pscychresns.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 42.Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/S0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- 43.Vonck K, Raedt R, Naulaerts J, et al. Vagus nerve stimulation…25 years later! What do we know about the effects on cognition? Neurosci Biobehav Rev. 2014;45:63–71. doi: 10.1016/j.neubiorev.2014.05.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.