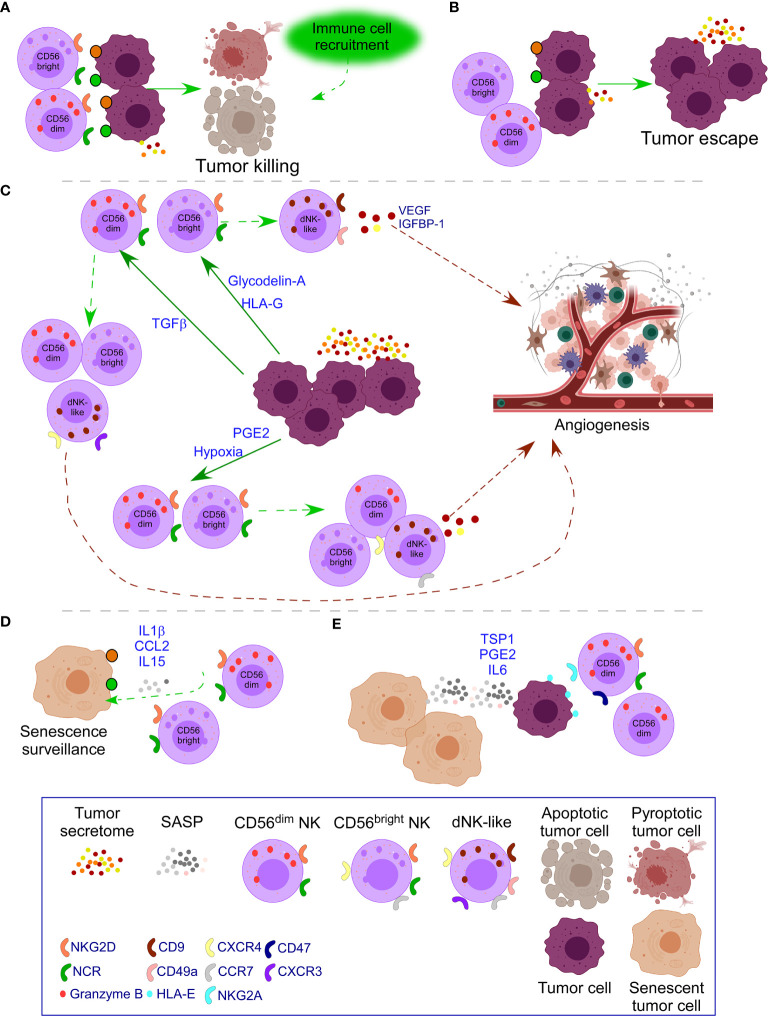
Figure 3.
Impact of tumor secretome in NK cell activity. (A) In healthy conditions, NK cells recognize transformed cells through ligands of NKG2D and the family of NCR receptors (NKp30, NKp44, NKp46) which are over-expressed in transformed cells. Pro-inflammatory forms of cell death attract additional immune cells to cooperate in the killing. (B) In some cases, tumor cells down-regulate ligands for NK cell receptors or the tumor microenvironment (TME) causes down-regulation of activating NK cell receptors leading to tumor escape with additional secretion of tumor secretome. (C) When tumor escape occurs, increased tumor secretome leads to additional changes in NK cells. Specifically, release of Glycodelin-A and HLA-G converts immunoregulatory CD56bright PB-NK cells into dNK-like cells. TGFβ converts both cytotoxic CD56dim and CD56bright NK cells into dNK-like cells; and down-regulates NK cell activating receptors limiting NK killer activity. PGE2 and hypoxia inhibit the expression of NK cell activating receptors and their functional maturation leading to suppressed NK cell cytotoxicity. Moreover, hypoxia, preserves immature CD56bright NK cells with expression of receptors of dNK cells, resembling to dNK-like cells. In all cases, dNK-like cells will activate angiogenesis processes. (D) Emergence of senescent tumor cells leads to SASP secretion that attracts NK cells to mediate their clearance. (E) When the number of senescent cells increases, the SASP also does, leading to inhibition of NK cell activity, through mechanisms, such as the interaction of HLA-E with the inhibitory receptor NKG2A in NK cells and binding of TSP1 with CD47 that inhibit NK cell activity. PGE2 and IL6 in the SASP also down-regulate NK cell activating receptors. Moreover, therapy-induced senescence in established tumors down-regulates NK cell activating receptors on mature NK cells and their ligands on tumor cells.