Figure 1.
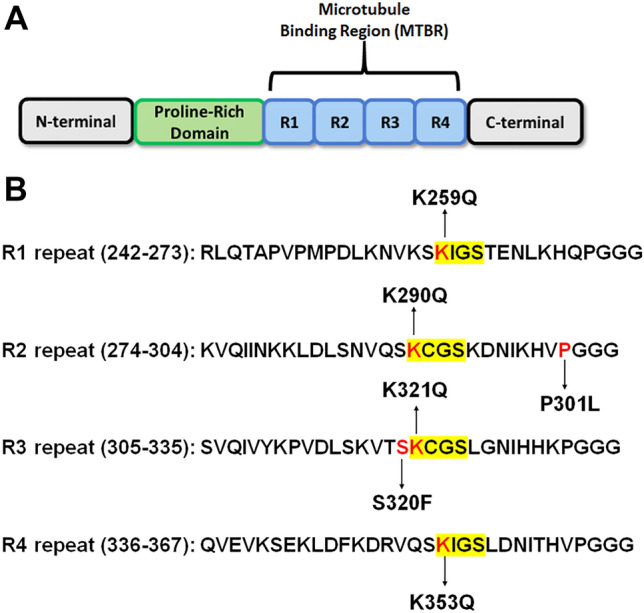
Tau protein structure and overview of pseudoacetylation and mutation sites. (A) Illustration shows the general structure of the tau protein with major domains: N-terminal domain, proline-rich region, MT-binding region consisting of four major repeats, and C-terminal domain. (B) Amino acid sequences of four MT binding repeats R1 to R4 are shown. Acetylation sites K259, K290, K321, and K353 are within conserved KCGS or KIGS motifs in each of the four major repeats, respectively, and numbered according to the 2N4R human tau isoform. Additionally, P301L and S320F MAPT missense mutations that are depicted were used as a model of intrinsic tau aggregation.
