Figure 6.
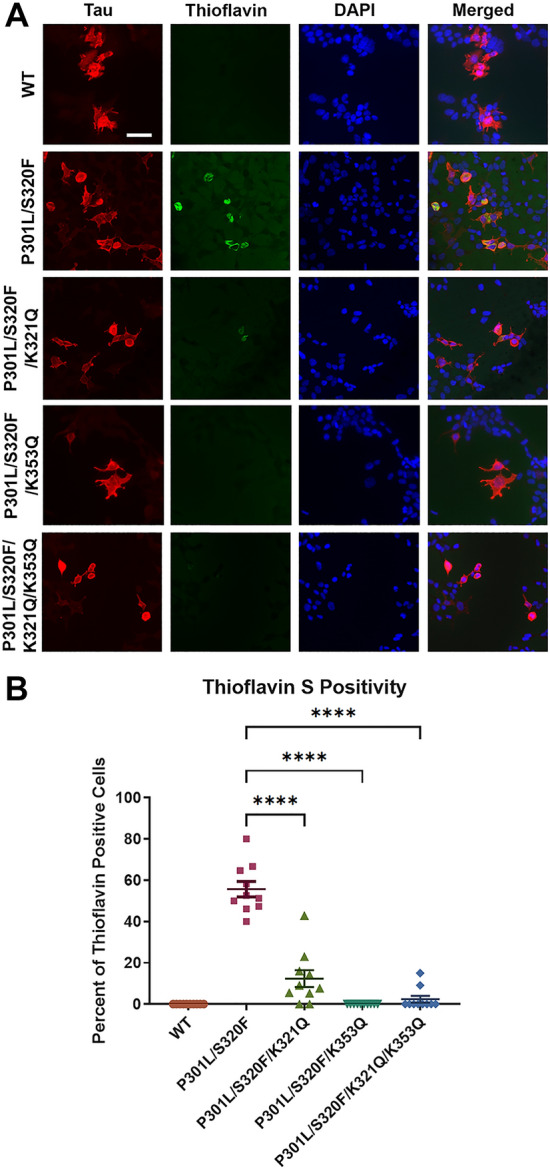
In the context of the P301L/S320F aggregation mutations, K321Q and K353Q acetylmimetics disrupt conformational amyloid structure as shown by reduced Thioflavin reactivity. (A) HEK293T cells were transfected to express WT, P301L/S320F, P301L/S320F/K321Q, P301L/S320F/K353Q, or P301L/S320F/K321Q/K353Q 0N4R tau. Cells were plated onto slides and labeled for fluorescence with DAPI for nuclei, 3026 antibody/Alexa 594 for total tau (red), and Thioflavin S for amyloid structure (green). (B) Graph shows the ratio of thioflavin positive to tau positive cells calculated from 10 different ×20 fields for each group. One-way ANOVA with Dunnett’s test was performed with N = 10 for WT tau and mutations. ****p < 0.0001. Scale bar = 50 µm.
