Abstract
Purpose
Coronavirus disease-2019 (COVID-19) is a novel respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2); there are few specific treatments. Convalescent plasma (CP), donated by people who have recovered from COVID-19, is an investigational therapy for severe or critically ill patients with COVID-19.
Materials and Methods
This retrospective cohort study evaluated the effectiveness of CP therapy in patients with severe or life-threatening cases of COVID-19 at two hospitals in Seoul, Korea, between May and September 2020. Clinical outcomes were evaluated in 20 patients with CP therapy in a descriptive manner. Additionally, the changes in cycle threshold (Ct) values of 10 patients with CP therapy were compared to those of 10 controls who had the same (±0.8) initial Ct values but did not receive CP.
Results
Of the 20 patients (mean age 66.6 years), 18 received high-dose oxygen therapy using mechanical ventilators or high-flow nasal cannulas. Systemic steroids were administered to 19 patients who received CP. The neutralizing antibody titers of the administered CP were between 1:80 and 1:10240. There were two ABO-mismatched transfusions. The World Health Organization ordinal scale score and National Institutes of Health severity score improved in half of the patients within 14 days. Those who received CP showed a higher increase in Ct values at 24 h and 72 h after CP therapy compared to controls with similar initial Ct values (p=0.002). No transfusion-related side effects were observed.
Conclusion
CP therapy may be a potential therapeutic option in severe or critically ill patients with COVID-19.
Keywords: Convalescent plasma treatment, COVID-19, neutralizing antibody, cycle threshold (Ct) value
INTRODUCTION
Coronavirus disease-2019 (COVID-19) is a novel respiratory infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). In the absence of effective confirmed therapy, empirical and experimental treatments for SARS have mainly taken place.1,2,3,4,5,6,7,8,9,10 Convalescent plasma (CP), donated by people who have recovered from COVID-19, is an investigational therapy for severe or critically ill patients with COVID-19. CP therapy has been used to treat patients with other viral infections, such as infections caused by the H1N1 influenza A virus, Argentinian mammarenavirus, Lassa mammarenavirus, Middle East respiratory syndrome coronavirus (MERS-CoV), SARS-CoV-1, and Ebola virus.11,12,13 The use of plasma from recovered patients has demonstrated clinical benefits in those with severe or life-threatening COVID-19, despite some methodological limitations in previous studies.14,15,16 Although the use of CP has shown favorable results in many cases, evidence supporting its use in the treatment of COVID-19 remains insufficient.17,18,19,20 Therefore, in this study, we aimed to evaluate the effectiveness and safety of CP therapy in severe or critically ill patients and the changes in viral load clearance based on cycle threshold (Ct) values over time between patients who received and did not receive CP.
MATERIALS AND METHODS
Study design and subjects
A retrospective study was conducted to evaluate the clinical outcomes of CP therapy in patients with severe or life-threatening COVID-19 at two hospitals in Seoul, Korea, between May and September 2020. Clinical outcomes were evaluated in 20 patients who received CP therapy in a descriptive manner. Simultaneously, the changes in Ct values of 10 patients who received CP transfusions were compared to those of 10 control patients who did not receive CP therapy. Patients with laboratory-confirmed cases of COVID-19, which were diagnosed using quantitative reverse transcriptase polymerase chain reaction (qRT-PCR), were eligible to receive CP treatment if they fulfilled the following criteria: 1) signed informed consent, 2) showed findings of pneumonia on imaging studies, and 3) had clinical features meeting the definitions of severe or life-threatening COVID-19. Severe COVID-19 was defined as respiratory distress with a respiratory rate of ≥30 breaths/min in the resting state, desaturation of ≤93% on room air, or arterial partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ≤300 mm Hg. Life-threatening COVID-19 was defined as respiratory failure requiring mechanical ventilation, septic shock, or multiple organ failure (organs other than the lungs) requiring intensive care unit monitoring.
Patients who were infected with SARS-CoV-2 but did not receive CP were enrolled in the control group for the comparison of viral load clearance. Within the ±0.8 range, these patients were conditioned to have the same initial Ct values as those in the CP group. Patients with CP were matched 1:1 with those who did not undergo CP with the same (±0.8) Ct value. The initial Ct values in both groups ranged between 19 and 34. The severity of the disease was not considered when matching the two groups.
Convalescent plasma therapy
All donors had been previously diagnosed with laboratory-confirmed COVID-19, and treated at the Seoul Medical Center. An information leaflet about CP donation was distributed to COVID-19 patients who were scheduled to be discharged. Those who announced intension to donate CP allowed the medical staffs to call them after discharge. After reconfirming their volition of donation on the line, plasmapheresis for each donor was scheduled. They had subsequently tested negative for SARS-CoV-2 and other viruses, such as hepatitis B virus, hepatitis C virus, and human immunodeficiency virus, and for syphilis at the time of plasma donation. After providing their written informed consent on the day of the plasmapheresis, the donors gave their CP. Afterwards, 500 mL of CP was collected from each donor by apheresis and stored in a frozen state. It was administered to the recipients after thawing. The ABO blood types of the patients were determined for potential compatibility with the CP donor. Except for two cases, each patient received two consecutive transfusions of 250 mL of ABO-compatible CP.
The levels of neutralizing antibodies against SARS-CoV-2 in donor plasma were measured by the plaque reduction neutralization test. Ten-fold serial dilutions of the virus stock were prepared in a virus dilution medium. A confluent monolayer of Vero cells was infected with serial dilutions of SARS-CoV-2 samples with an unknown starting concentration. After adsorption, an immobilizing overlay was used to cover the infected monolayer to prevent virus spread and restrict virus growth to the foci of cells at the sites of initial infection. During incubation, the zones of cell death developed as viral infection and replication were restricted to the surrounding monolayer, leading to plaque formation. After incubation, the cells were stained to enhance contrast between plaques and the uninfected monolayer. Plaques were then enumerated and used to calculate the titer of the infectious virus in the specimen.
Data collection
Data on the following parameters were collected: demographics; days to admission from symptom onset; therapeutic modalities including high-dose oxygen therapy, antiviral therapies, and steroids; clinical parameters including National Institutes of Health (NIH) severity scores, World Health Organization (WHO) ordinal scale scores, and PaO2/FiO2 ratios; laboratory parameters including white blood cell counts, lymphocyte counts, C-reactive protein (CRP) levels, and Ct values for SARS-CoV-2; and findings of chest imaging studies. Remdesivir was administered for 5 days. Steroids treatment for more than 1 day was counted to therapeutic modalities. Nasopharyngeal swabs (NP) and sputum samples were serially collected from 20 patients receiving CP transfusions 1 day before the CP therapy and on the 1st and 3rd day after CP therapy. The same types of specimens were also serially collected from COVID-19 patients who did not receive CP therapy on the admission day, as well as on the 1st and 3rd day after admission. qRT-PCR for SARS-CoV-2 was performed using NP and sputum specimens. Real-time RT-PCR assay targeting the three genes (RdRp, N, and E genes) of SARS-Cov-2 was performed with a Seegene Kit (Allplex 2019-nCoV Assay kit, Seegene, Seoul, Korea).
Clinical outcomes and changes in viral loads within a 28-day period were evaluated. The improvement of disease severity scale scores, change in the PaO2/FiO2 ratio, and any adverse events, including transfusion-related adverse events, were also evaluated.
The WHO ordinal scale scores were classified as follows: 8 points, death; 7 points, hospitalization plus extracorporeal membrane oxygenation or invasive mechanical ventilation; 6 points, hospitalization and the requirement of high-flow nasal cannulas (HFNC) or non-invasive ventilation; 5 points, hospitalization with any supplemental oxygen therapy; 4 points, hospitalization with ongoing medical care but without oxygen therapy; 3 points, hospitalization without oxygen therapy and ongoing medical care, 2 points; ambulation with limited activity or with home oxygen support; and 1 point, ambulation with unlimited activity.
Disease severity according to the NIH severity scale was classified as follows:
1) Asymptomatic or pre-symptomatic infection: individuals testing positive for SARS-CoV-2 but not showing symptoms
2) Mild illness: individuals with any of the various signs and symptoms of COVID-19 but without shortness of breath, dyspnea, or abnormal chest imaging findings
3) Moderate illness: individuals with evidence of lower respiratory disease on clinical assessment or imaging and oxygen saturation (SpO2) of ≥94% on room air at sea level
4) Severe illness: individuals with a respiratory rate of >30 breaths per minute, SpO2 of <94% on room air at sea level, a PaO2/FiO2 ratio of ≤300 mm Hg, or lung infiltrates of >50%
5) Critical illness: individuals with respiratory failure, septic shock, and/or multiple organ dysfunction
Statistical analysis
All variables are expressed as the mean (range) or number (percentage), unless otherwise indicated. The longitudinal data of Ct values in the matching groups were compared using a linear mixed model. Statistical significance was set at a p value<0.05. Statistical analysis was performed using the Statistical Package for Social Sciences software (version 25.0; IBM Corp., Armonk, NY, USA).
Ethics statement
This study was approved by the Institutional Review Board of the Yonsei University Health System Clinical Trial Center (4-2020-0263). Informed consent forms were signed by the participants who were willing to receive CP therapy and participate in the necessary research studies.
RESULTS
Clinical outcomes in patients receiving convalescent plasma therapy
Table 1 shows the clinical characteristics of patients who received CP therapy. The mean age was 66.6 years, and the sex ratio (male: female) was 11:9. Of all patients, 65% were critically ill according to the NIH severity criteria. Findings of pneumonia were observed on the initial chest imaging studies of all 20 patients. All patients had a PaO2/FiO2 ratio ≤300 mm Hg. Eighteen patients (90%) required supplemental high-dose oxygen via mechanical ventilators or HFNC. Nineteen patients (95%) received systemic steroids therapy. Five patients initially received combination therapy with steroids and remdesivir.
Table 1. Baseline Characteristics of Patients with CP Therapy (n=20).
| Characteristics | Values | |
|---|---|---|
| Mean age, yr (range) | 66.6 (44–87) | |
| Male | 11 (55) | |
| Underlying diseases | ||
| None | 8 (40) | |
| Hypertension only | 4 (20) | |
| Cardiovascular diseases | 2 (10) | |
| Diabetes mellitus | 3 (15) | |
| Malignancy | 1 (5) | |
| Pneumonia on chest image | 20 (100) | |
| O2 supply | ||
| No oxygen | 1 (5) | |
| Nasal O2 cannula | 1 (5) | |
| High flow nasal cannula | 5 (25) | |
| Mechanical ventilator | 13 (65) | |
| Admission to CP, days | 5.55 (±3.38) | |
| Symptom onset to CP, days | 11.5 (±6.98) | |
| NIH severity | ||
| Moderate | 1 (5) | |
| Severe | 6 (30) | |
| Critical | 13 (65) | |
| Lymphopenia | 16 (80) | |
| CRP, mg/dL | 10.26 (±6.99) | |
| LDH, U/L | 455.37 (±130.8) | |
| Combined treatments | ||
| Remdesivir | 5 (25) | |
| Systemic steroid | 19 (95) | |
| Neutralizing antibody titers of used plasma | ||
| Unknown | 2 (10) | |
| 1:80 | 2 (10) | |
| 1:160 | 5 (25) | |
| 1:320 | 4 (20) | |
| 1:1280 | 4 (20) | |
| 1:2560 | 2 (10) | |
| 1:10240 | 1 (5) | |
CP, convalescent plasma; NHI, National Institute of Health; CRP, C-reactive protein; LDH, lactate dehydrogenase; SD, standard deviation.
Values are expressed as the n (%) or mean (±SD).
The average duration from admission to convalescent therapy was 5.55 days, and that from symptom onset to CP was 11.5 days. In the initial laboratory assessment, lymphopenia was noted in 15 patients. The mean levels of CRP and lactate dehydrogenase (LDH) were 10.39±7.11 and 455.36±125.88, respectively. The neutralizing antibody titers of the administered CP were between 1:80 and 1:10240. Eighty percent of the donated CP samples had a neutralizing antibody titer of ≥1:160. More significant Ct value change was not associated with a higher neutralizing antibody titer. Overall, there was no prognostic difference according to the neutralizing antibody titers.
The PaO2/FiO2 ratio was improved among all survivors in 14 days (mean PaO2/FiO2 ratio on day 1: mean PaO2/FiO2 ratio on day 14=189.73:232.34) (Table 2).
Table 2. Treatment Outcomes of Patients with Convalescent Plasma Therapy (n=20).
| Outcomes | Number of patients (%) | |
|---|---|---|
| Mortality | ||
| 7-day mortality | 1 (5) | |
| 14-day mortality | 2 (10) | |
| 28-day mortality | 4 (20) | |
| Overall mortality | 5 (25) | |
| Improvement in severity within 7 days | ||
| WHO ordinal scale | 8 (40) | |
| NIH severity | 5 (25) | |
| Improvement in severity within 14 days | ||
| WHO ordinal scale | 10 (50) | |
| NIH severity | 10 (50) | |
| Improvement of P/F ratio among survivors | 15 (100) | |
| Transfusion-related adverse events | 0 (0) | |
WHO, World Health Organization; NIH, National Institute of Health.
The WHO ordinal scale score and NIH severity score were improved in half of the patients within 14 days of CP transfusion. Five patients (25%) showed a reduction of 2 points within 14 days. The overall mortality rate among patients who received CP therapy was 25%. One patient (5%) died within 7 days of plasma transfusion (on day 4 post-transfusion). The other four deaths occurred on days 11, 15, 28, and 60 after transfusion. All patients died due to COVID-19-related complications.
Among the 20 CP recipients, two patients received ABO-mismatched transfusions. One recipient with ABO type O received CP from a donor with blood type B, and the other recipient with ABO type AB had CP from a donor with blood type A. No suspected transfusion-related adverse events were reported.
Comparison of viral load clearance between patients who did and did not receive convalescent plasma
Table 3 shows the baseline characteristics of the 10 patients who received CP therapy and were matched with 10 controls based on similar initial Ct values. The most patients in both groups were in their 60s. All 10 patients who received CP were severely ill. In contrast, none of the 10 patients in the control group were severely ill. Four patients in the control group required oxygen support via nasal cannula and were treated with remdesivir for 5 days, but none of them received steroids. Patients who received CP required high-dose oxygen therapy. Sixty percent of the patients in the control group were treated without oxygen supplementation. All 20 patients initially had lymphopenia. The average CRP and LDH values were higher in the CP group than in the control group (Table 3).
Table 3. Baseline Characteristics of Patients with and without CP Who Had Similar Initial Ct Values.
| Characteristics | With CP (n=10) | Without CP (n=10) | |
|---|---|---|---|
| Mean age, yr (range) | 65.3 (44–83) | 62.8 (31–81) | |
| Male | 7 (70) | 5 (50) | |
| Underlying diseases | |||
| None | 3 (30) | 5 (50) | |
| Hypertension only | 1 (10) | 0 (0) | |
| Cardiovascular diseases | 1 (10) | 1 (10) | |
| Diabetes mellitus | 2 (20) | 2 (20) | |
| Malignancy | 1 (10) | 0 (0) | |
| Pneumonia on chest image | 10 (100) | 6 (60) | |
| O2 supply | |||
| No oxygen | 0 (0) | 6 (60) | |
| Nasal O2 cannula | 0 (0) | 4 (40) | |
| High flow nasal cannula | 4 (40) | 0 (0) | |
| Mechanical ventilator | 6 (60) | 0 (0) | |
| NIH severity | |||
| Moderate | 0 (5) | 6 (60) | |
| Severe | 3 (30) | 0 (0) | |
| Critical | 7 (70) | 0 (0) | |
| Remdesivir | 3 (30) | 4 (40) | |
| Steroids | 10 (100) | 0 (0) | |
| Lymphopenia | 6 (60) | 6 (60) | |
| CRP, mg/dL | 10.23 (±8.4) | 3.56 (±4.5) | |
| LDH, IU/L | 481.44 (±119.14) | 362.4 (±87.98) | |
NIH, National Institute of Health; CP, convalescent plasma; Ct, cycle threshold value; CRP, C-reactive protein; LDH, lactate dehydrogenase; SD, standard deviation.
Values are expressed as the n (%) or mean (±SD).
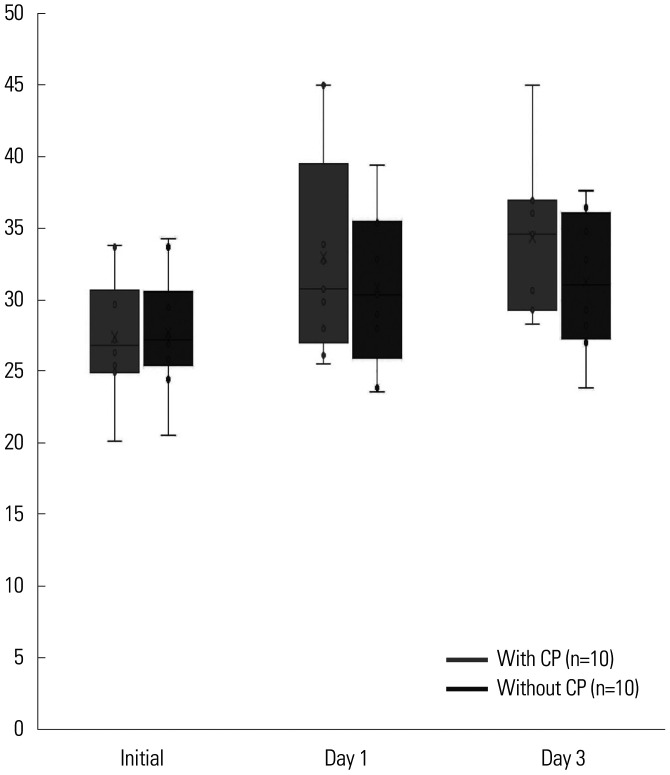
The initial Ct values of the E gene from nasopharyngeal specimens of patients who received CP ranged from 20.09 to 35. At 24 h after CP therapy, Ct values of the E gene increased to range between 26.19 and 45. On comparing the Ct values of 10 patients at 24 h and 72 h after CP therapy with 10 control patients who did not receive CP therapy, the viral load clearance was higher in patients who received CP (p=0.002). The comparison of Ct values of the E gene of two groups are shown in Fig. 1. In many study participants, Ct values were not measured consistently on the 5th, 7th, 14th, and 28th day. For this reason, the Ct values were indicated until the 3rd day in Fig. 1.
Fig. 1. Comparison of viral loads clearance (E-gene) between patients with and without convalescent plasma (CP) (p=0.002). p value<0.05 was considered to indicated statistical significance.
The average initial Ct values of all 20 patients with CP was 27.2. The mean Ct values were increasing day after day, eventually exceeding 35 on the 7th day after CP, and indicated low infectivity. The average initial Ct value of 10 patients who did not belong to the comparison group of Ct values was 26.14. The average initial Ct value of other 10 patients in the comparison group was 28.26. On the 1st day, the average Ct values among these patients were 33.4 and 31.2, respectively. Of the total five cases of mortality, four cases occurred in the selected group.
DISCUSSION
Numerous studies have analyzed the effectiveness of CP therapy for COVID-19. Some patients showed improved clinical outcomes. Increased oxygen saturation, recovery from lymphopenia, and reduced inflammation were reported after CP treatment in small studies.21,22,23 In a case series of five critically ill patients, Shen, et al.19 showed decreased viral loads and improved clinical conditions within days of plasma infusion. In several studies, a rapid reduction in viral load was reported.24,25 In this study, the SARS-CoV-2 load in the respiratory tract decreased at a higher rate in patients who received CP in a subgroup analysis involving a comparison with 10 patients who did not receive CP therapy. Viral loads were significantly lower at 24 h and 72 h after CP therapy. As assessed by the Ct values, the viral load declined within days of CP treatment, and the clinical condition of these patients improved. This provides evidence supporting CP transfusion as a valuable intervention in severe or critically ill COVID-19 patients.
However, many studies have demonstrated no differences in mortality or disease severity between the treatment and control groups. In the ConCOVID study, an open-label randomized clinical trial of CP therapy versus the standard of care for hospitalized COVID-19 patients, which was conducted in 14 hospitals in the Netherlands, no differences in mortality or disease severity were observed between the groups on day 15.26 In the PLACID Trial, an open-label randomized clinical trial with 464 participants, no superiority of CP therapy over the standard care was observed in terms of disease progression and 28-day mortality.27 In another randomized trial with 334 patients who were assigned in a 2:1 ratio to receive CP or a placebo, no meaningful distinctions were found in the clinical status or overall mortality between the two groups.28 Similar to these studies, no significant difference in outcomes with CP therapy were observed in the latest large-scale studies.29,30 The efficacy of CP therapy for COVID-19 remains controversial. Further research and experience regarding CP therapy are required to confirm its effectiveness.
In several studies, the conditions and settings in which CP was more effective in COVID-19 patients were investigated. First, the timing of transfusion after symptom onset was evaluated to optimize the effectiveness of CP therapy in multiple studies. Some studies have reported that better outcomes were associated with early transfusion therapy after the onset of symptoms.31,32,33 Joyner, et al.34 showed a significant reduction in mortality after the transfusion of CP with high antibody levels within 3 days of COVID-19 diagnosis. In addition, Joyner, et al.35 reported in a different study that the risk of death within 30 days was lower in patients who did not receive mechanical ventilation but were treated with CP containing higher anti-SARS-CoV-2 antibody levels. One retrospective case-control study suggested a trend toward the benefit of CP therapy among patients who were not intubated.15 In a randomized controlled trial with elderly patients, it was reported that CP therapy in older patients reduced the risk of progression to severe respiratory disease when CP with high titers of antibodies was administered within 72 h of symptom onset.36 Overall, treatment using CP with high titers of antibodies was beneficial against SARS-CoV-2 infection among patients with a shorter duration of symptoms and those who were not intubated. Unfortunately, this study failed to show the correlation between better prognosis and higher neutralizing antibody titer of donors. The number of recipients was not large enough to evaluate the positive relationship between outcomes, and higher antibody levels in CP and early CP administration. A randomized trial with a larger number of recipients at multiple centers is needed to draw definitive conclusions about the efficacy of CP for the treatment of COVID-19.
The safety of CP treatment in severe COVID-19 patients has been a major concern in various studies. Serious transfusion-related adverse events, such as allergic transfusion reactions, hemolytic transfusion reactions, transfusion-related acute lung injury, and transfusion-associated circulatory overload, can occur during CP therapy. In most studies, severe adverse events were uncommon.15,20,21,37,38,39 In this study, there was no evidence of serious adverse events or complications related to CP therapy, although ABO-mismatched transfusion occurred in two cases. This suggests that CP therapy is a safe method for treating COVID-19.
The antiviral remdesivir is known to help improve early symptoms of COVID-19, and steroids are known to lower mortality in severely ill patients with COVID-19. In this study, remdesivir treatment was performed in almost the same number of patients in both groups. However, all 10 patients in CP group were treated with steroids, but none of the patients in the control group received steroids. The possibility could be considered that steroids may have contributed to lowering the viral loads. Spagnuolo, et al.40 showed that SARS-CoV-2 clearance was not associated with corticosteroid use in their study with 280 moderate or severe COVID-19 patients. It is still hasty to conclude that the effects of steroids on viral loads are insignificant, and additional research data regarding this topic should be accumulated.
Similar to the majority of studies on CP therapy for severely ill COVID-19 patients, this study had a number of critical limitations. First, this was a small study involving two healthcare centers with a non-randomized design. Therefore, it is unclear whether the clinical improvement observed in the 20 patients who received CP would have occurred without this treatment. Second, since nearly all of the patients were also treated with other medications, such as steroids or remdesivir, the extent to which the combined drug therapies affected the outcomes remains unclear. Third, the size of the donor pool was small. A larger donor pool would provide opportunities for severely ill patients to receive high-titer CP earlier in the disease course. Furthermore, the number of study participants were too small to indicate the correlation between clinical outcomes and higher antibody levels in CP and early CP administration among patients with or without ventilation care. Finally, matching with the control group was only based on similar initial Ct values. As other factors were not considered for matching, selection bias cannot be excluded. An important potential confounder in this analysis was the difference in disease severity between the two groups. This study was not objective enough to compare the changes in Ct values between the two groups. Considering these limitations, additional studies are needed to confirm the beneficial findings and draw more definitive conclusions about the efficacy of CP transfusion for the treatment of COVID-19.
In conclusion, CP therapy might be a safe and potentially effective therapeutic option for improving the outcomes of severely ill COVID-19 patients.
Footnotes
The authors have no potential conflicts of interest to disclose.
- Conceptualization: JunYong Choi.
- Data curation: YunSuk Cho and JunYong Choi.
- Formal analysis: YunSuk Cho and JunYong Choi.
- Investigation: YunSuk Cho, YuJin Sohn, JongHoon Hyun, YaeJee Baek, MooHyun Kim, JungHo Kim, JinYoung Ahn, SuJin Jeong, NamSu Ku, Joon-Sup Yeom, MiYoung Ahn, DongHyun Oh, and JunYong Choi.
- Methodology: YunSuk Cho and JunYong Choi.
- Project administration: JunYong Choi.
- Resources: YunSuk Cho, MiYoung Ahn, Dong-Hyun Oh, JaePhil Choi, SinYoung Kim, and KyoungHwa Lee.
- Software: YunSuk Cho.
- Supervision: YuJin Sohn, JongHoon Hyun, YaeJee Baek, MooHyun Kim, JungHo Kim, JinYoung Ahn, SuJin Jeong, Nam-Su Ku, Joon-Sup Yeom, MiYoung Ahn, DongHyun Oh, JaePhil Choi, SinYoung Kim, KyoungHwa Lee, YongGoo Song, and JunYong Choi.
- Validation: YunSuk Cho and SinYoung Kim.
- Visualization: YunSuk Cho.
- Writing—original draft: YunSuk Cho.
- Writing—review & editing: YunSuk Cho, YuJin Sohn, and JunYong Choi.
- Approval of final manuscript: all authors.
References
- 1.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of COVID-19-final report. N Engl J Med. 2020;383:1813–1826. doi: 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pan H, Peto R, Henao-Restrepo AM, Preziosi MP, Sathiyamoorthy V, Abdool Karim Q, et al. Repurposed antiviral drugs for COVID-19—interim WHO solidarity trial results. N Engl J Med. 2021;384:497–511. doi: 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqi HK, Mehra MR. COVID-19 illness in native and immuno-suppressed states: a clinical-therapeutic staging proposal. J Heart Lung Transplant. 2020;39:405–407. doi: 10.1016/j.healun.2020.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Furtado RHM, Berwanger O, Fonseca HA, Corrêa TD, Ferraz LR, Lapa MG, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Omura S, Crump A. Ivermectin: panacea for resource-poor communities? Trends Parasitol. 2014;30:445–455. doi: 10.1016/j.pt.2014.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Jiang S, Hillyer C, Du L. Neutralizing antibodies against SARS-CoV-2 and other human coronaviruses. Trends Immunol. 2020;41:355–359. doi: 10.1016/j.it.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis. 2020;11:462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Florescu DF, Kalil AC, Hewlett AL, Schuh AJ, Stroher U, Uyeki TM, et al. Administration of brincidofovir and convalescent plasma in a patient with Ebola virus disease. Clin Infect Dis. 2015;61:969–973. doi: 10.1093/cid/civ395. [DOI] [PubMed] [Google Scholar]
- 12.Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. 2011;52:447–456. doi: 10.1093/cid/ciq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhou B, Zhong N, Guan Y. Treatment with convalescent plasma for influenza A (H5N1) infection. N Engl J Med. 2007;357:1450–1451. doi: 10.1056/NEJMc070359. [DOI] [PubMed] [Google Scholar]
- 14.Bakhtawar N, Usman M, Khan MMU. Convalescent plasma therapy and its effects on COVID-19 patient outcomes: a systematic review of current literature. Cureus. 2020;12:e9535. doi: 10.7759/cureus.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu STH, Lin HM, Baine I, Wajnberg A, Gumprecht JP, Rahman F, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med. 2020;26:1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- 16.Salazar E, Christensen PA, Graviss EA, Nguyen DT, Castillo B, Chen J, et al. Treatment of coronavirus disease 2019 patients with convalescent plasma reveals a signal of significantly decreased mortality. Am J Pathol. 2020;190:2290–2303. doi: 10.1016/j.ajpath.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two COVID-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. 2020;35:e149. doi: 10.3346/jkms.2020.35.e149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pei S, Yuan X, Zhang Z, Yao R, Xie Y, Shen M, et al. Convalescent plasma to treat COVID-19: Chinese strategy and experiences. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.04.07.20056440v1.
- 19.Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically ill patients with COVID-19 with convalescent plasma. JAMA. 2020;323:1582–1589. doi: 10.1001/jama.2020.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. 2020;92:1890–1901. doi: 10.1002/jmv.25882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci U S A. 2020;117:9490–9496. doi: 10.1073/pnas.2004168117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Erkurt MA, Sarici A, Berber İ, Kuku İ, Kaya E, Özgül M. Life-saving effect of convalescent plasma treatment in COVID-19 disease: clinical trial from eastern Anatolia. Transfus Apher Sci. 2020;59:102867. doi: 10.1016/j.transci.2020.102867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown BL, McCullough J. Treatment for emerging viruses: convalescent plasma and COVID-19. Transfus Apher Sci. 2020;59:102790. doi: 10.1016/j.transci.2020.102790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yeh KM, Chiueh TS, Siu LK, Lin JC, Chan PK, Peng MY, et al. Experience of using convalescent plasma for severe acute respiratory syndrome among healthcare workers in a Taiwan hospital. J Antimicrob Chemother. 2005;56:919–922. doi: 10.1093/jac/dki346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. The feasibility of convalescent plasma therapy in severe COVID-19 patients: a pilot study. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.03.16.20036145v1.
- 26.Gharbharan A, Jordans CCE, Geurtsvankessel C, den Hollander JG, Karim F, Mollema FPN, et al. Convalescent plasma for COVID-19. A randomized clinical trial. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.07.01.20139857v1.
- 27.Agarwal A, Mukherjee A, Kumar G, Chatterjee P, Bhatnagar T, Malhotra P. Convalescent plasma in the management of moderate COVID-19 in adults in India: open label phase II multicentre randomised controlled trial (PLACID Trial) BMJ. 2020;371:m3939. doi: 10.1136/bmj.m3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simonovich VA, Burgos Pratx LD, Scibona P, Beruto MV, Vallone MG, Vázquez C, et al. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2021;384:619–629. doi: 10.1056/NEJMoa2031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. Dexamethasone in hospitalized patients with COVID-19. N Engl J Med. 2021;384:693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chai KL, Valk SJ, Piechotta V, Kimber C, Monsef I, Doree C, et al. Convalescent plasma or hyperimmune immunoglobulin for people with COVID-19: a living systematic review. Cochrane Database Syst Rev. 2020;10:CD013600. doi: 10.1002/14651858.CD013600.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. 2004;10:676–678. doi: 10.1111/j.1469-0691.2004.00956.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cheng Y, Wong R, Soo YO, Wong WS, Lee CK, Ng MH, et al. Use of convalescent plasma therapy in SARS patients in Hong Kong. Eur J Clin Microbiol Infect Dis. 2005;24:44–46. doi: 10.1007/s10096-004-1271-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mair-Jenkins J, Saavedra-Campos M, Baillie JK, Cleary P, Khaw FM, Lim WS, et al. The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis. 2015;211:80–90. doi: 10.1093/infdis/jiu396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joyner MJ, Senefeld JW, Klassen SA, Mills JR, Johnson PW, Theel ES, et al. Effect of convalescent plasma on mortality among hospitalized patients with COVID-19: initial three-month experience. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.08.12.20169359v1.
- 35.Joyner MJ, Carter RE, Senefeld JW, Klassen SA, Mills JR, Johnson PW, et al. Convalescent plasma antibody levels and the risk of death from COVID-19. N Engl J Med. 2021;384:1015–1027. doi: 10.1056/NEJMoa2031893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Libster R, Pérez Marc G, Wappner D, Coviello S, Bianchi A, Braem V, et al. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021;384:610–618. doi: 10.1056/NEJMoa2033700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Joyner MJ, Wright RS, Fairweather D, Senefeld JW, Bruno KA, Klassen SA, et al. Early safety indicators of COVID-19 convalescent plasma in 5000 patients. J Clin Invest. 2020;130:4791–4797. doi: 10.1172/JCI140200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin C, Gu J, Yuan Y, Long Q, Zhang Q, Zhou H, et al. Treatment of six COVID-19 patients with convalescent plasma. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.05.21.20109512v2.
- 39.Salazar E, Perez KK, Ashraf M, Chen J, Castillo B, Christensen PA, et al. Treatment of COVID-19 patients with convalescent plasma in Houston, Texas. [accessed on 2021 February 10]. Available at: https://www.medrxiv.org/content/10.1101/2020.05.08.20095471v1.
- 40.Spagnuolo V, Guffanti M, Galli L, Poli A, Querini PR, Ripa M, et al. Viral clearance after early corticosteroid treatment in patients with moderate or severe COVID-19. Sci Rep. 2020;10:21291. doi: 10.1038/s41598-020-78039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]