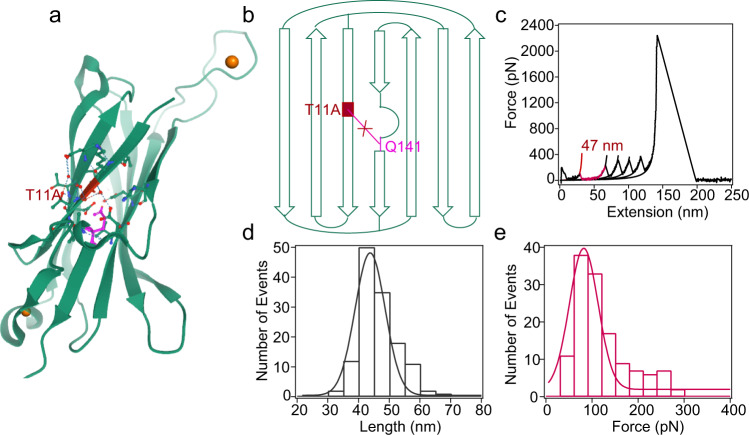
Fig. 2. Mechanical unfolding of the mutated C1 domain without the ester bond.
a Thr-11 of C1 was mutated to Ala to eliminate the ester bond to yield the C1T11A mutant. b Topology of C1T11A. The ester bond cannot form in the mutant. c Representative single-molecule force–extension curve of stretching Fgβ-(GB1)2-C1T11A-(GB1)2-cys following the same experimental protocol shown in Fig. 1d. Each peak was fitted by worm-like chain (WLC) model of polymer elasticity. The peak with a ΔLc of 47 nm corresponds to the unfolding of C1T11A, the next four peaks correspond to the unfolding of GB1 domains, and the last peak corresponds to the rupture of the Fgβ/SdrG complex. d Histogram of contour length increment for C1 unfolding is centered ~47 nm. e Unfolding force histogram of C1 domain at a pulling speed of 1.6 μm s−1 measures an average unfolding force of 92 ± 41 pN (n = 129, total number of C1 unfolding events). Fitting the force distribution with Monte Carlo simulation65 results in a Δxu of 0.27 nm and α0 of 0.35 s−1 (Supplementary Fig. 8). This experiment was repeated three times independently with similar results. Source data are provided as a Source Data file.