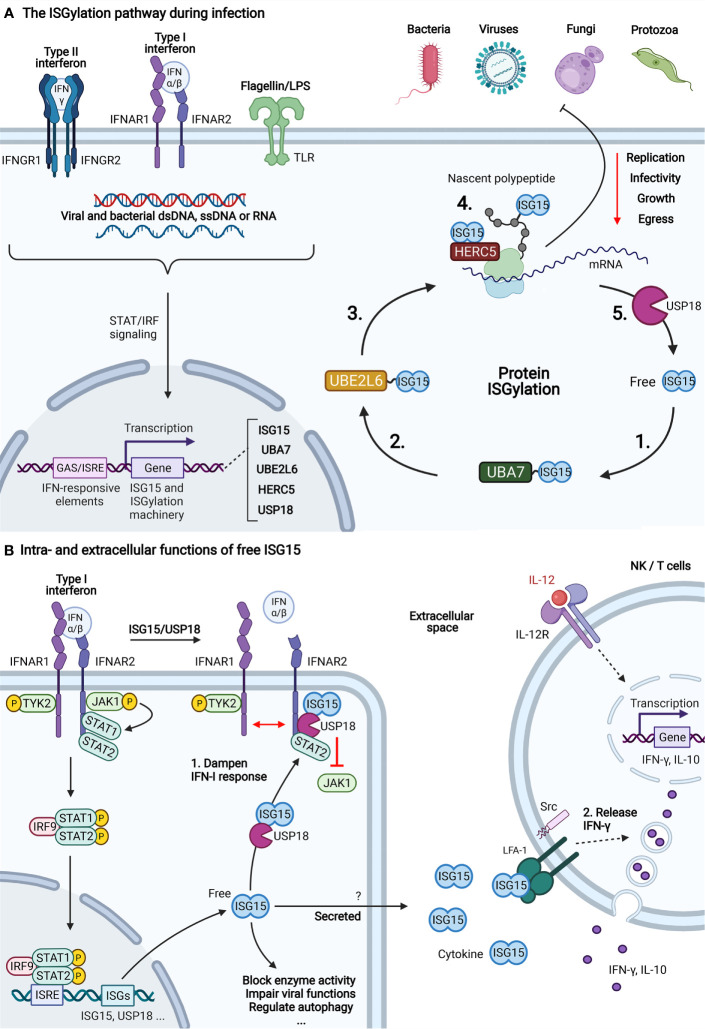
Figure 1.
The three functions of ISG15. (A) The ISGylation pathway during infection. After detecting the presence of an intra- or extracellular pathogen, several antimicrobial signaling pathways lead to the expression of ISGs, including ISG15 and its conjugation machinery. The covalent attachment of ISG15 to substrate proteins, also called ISGylation, relies on the activities of an E1 (UBA7) (1), E2 (UBE2L6) (2) and E3 (hHERC5/mHERC6) (3) enzyme. The product of the ISG15-conjugation pathway is an ISGylated host or viral protein (4). Target modification by ISG15 has a widespread negative effect on the replication, growth, egress and infectivity of four major classes of pathogens, including viruses, bacteria, fungi and protozoa (1). Deconjugation is catalyzed by USP18 which releases ISG15 from its substrate (5) (6). (B) Intra- and extracellular functions of free ISG15. Apart from its activity as a ubiquitin-like conjugate, ISG15 also exists in a free form with functions both inside and outside the cell. (1) Type I interferon (IFN-I) signaling is the main pathway for induction of ISG15 and the ISGylation machinery, including the deISGylase USP18. Binding of IFN-I to the dimeric IFN-I receptor (IFNAR1/2) results in recruitment and activation of Janus activated kinases (JAKs) and tyrosine kinase 2 (TYK2). Consequently, the JAKs phosphorylate STAT1 and STAT2 which dimerize and form a three-protein complex with IRF9. This complex translocates to the nucleus where it binds to IFN-responsive regulatory elements (ISRE) to initiate transcription of IFN-stimulated genes (ISGs). Among these genes is Usp18, which, in addition to its enzymatic activity, also functions as a negative regulator of IFN-I signaling by binding to IFNAR2 (7). There, it prevents dimerization of the receptor and blocks recruitment of JAKs which puts a brake on IFN-I-signaling (8). There, it prevents dimerization of the receptor and blocks recruitment of JAKs which puts a brake on IFN-I-signaling (8). In humans, this function depends on direct interaction with ISG15 which protects USP18 from proteasomal degradation (3). Apart from its role in IFN-I signaling, free ISG15 also blocks the activity of certain enzymes, impairs viral functions by sequestering viral proteins, regulates autophagy (9). (2) When ISG15 is not conjugated to other proteins, it also becomes secreted through an unknown non-canonical secretion pathway (10, 11). In the extracellular space, it acts as a cytokine for NK and T cells where it binds to LFA-1 and induces the secretion of IFN-γ and IL-10 from secretory granules (5). The mechanism relies on a synergy between IL-12 and ISG15 where IL-12 triggers the expression of IFN-γ and ISG15 promotes the secretion of IFN-γ through downstream activation of SRC kinases. In addition, ISG15 was shown to enhance secretion of other pro-inflammatory cytokines such as CXCL1, CXCL5, IL-1 and IL-6 (12, 13). Figure created with BioRender.com.