Fig. 1.
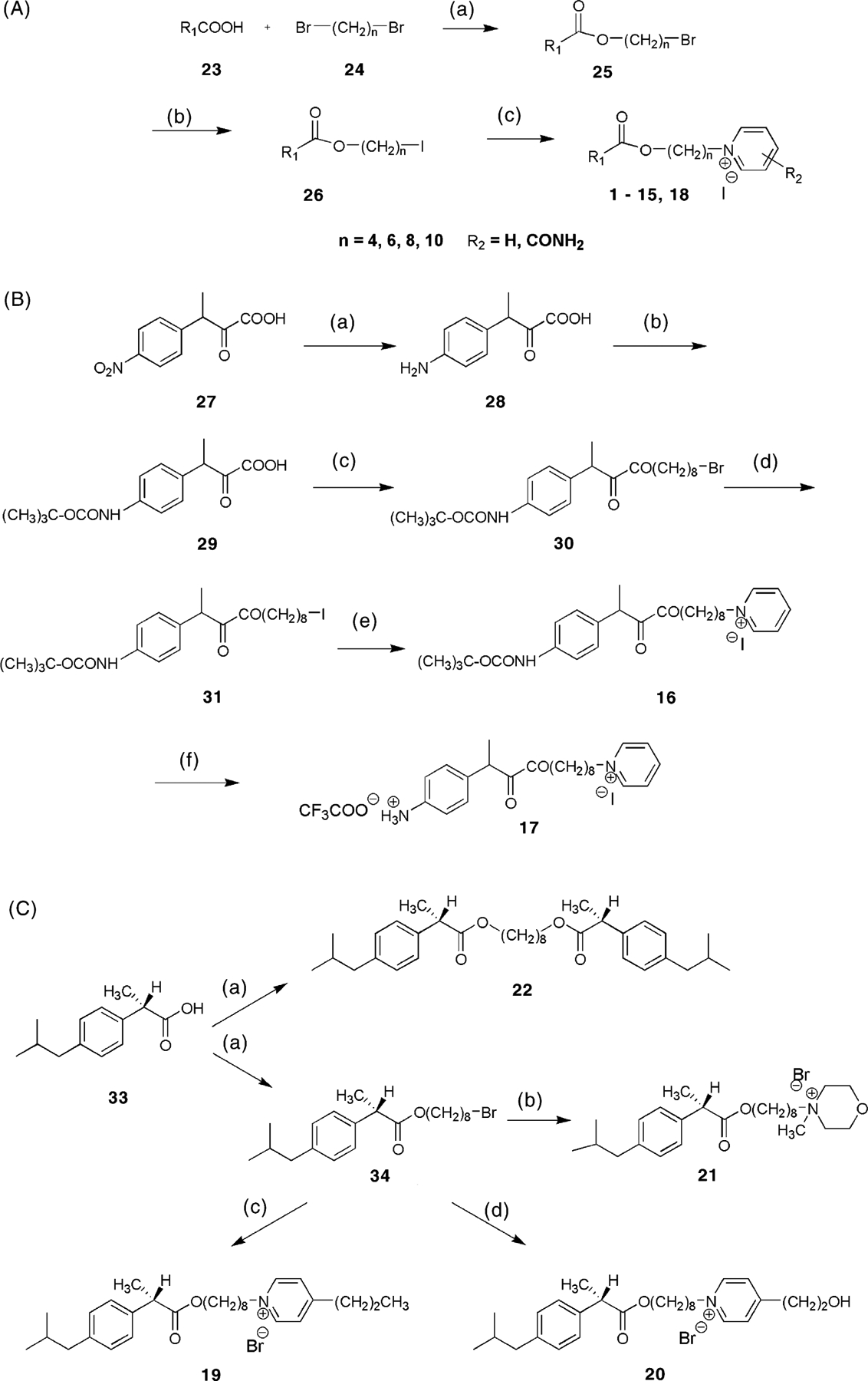
Chemical strategies for syntheses of MRS2481 and analogs. (A) Preparation of analogues of variable spacer chain lengths and variable substitution of the pyridinium moiety. For the structure of R1, refer to Table 1. Reagents: (a) benzyltrimethylammonium hydroxide, tetrabutylammonium iodide at r.t. for 2 days; (b) sodium iodide in acetone; (c) pyridine derivative in acetone at 50 °C for 3 days. (B) Preparation of compound 17, which contains a chemically reactive aryl amino group. Reagents: (a) tin, acetic acid, HCl at 100 °C for 1.5 h; (b) di-t-butyl-dicarbonate in methanol at 45 °C for 1 h; (c) benzyltrimethylammonium hydroxide, tetrabutylammonium iodide at r.t. for 2 days; (d) sodium iodide in acetone; (e) pyridine in acetone at 50 °C for 3 days; (f) trifuoroacetic acid. (C) Preparation of 4-substituted pyridinium salts 19 and 20 and non-pyridinium derivatives, including the symmetric diester 22. Reagents: (a) 1,8-dibromooctane, benzyltrimethylammonium hydroxide, tetrabutylammonium iodide at r.t. for 3 days; (b) N-methylmorpholine, tetrabutylammonium iodide in acetone at 50 °C for 2 days; (c) 4-propyl-pyridine, tetrabutylammonium iodide in acetone at 50 °C for 3 days; (d) 4-(2-hydroxyethyl)-pyridine, tetrabutylammonium iodide in acetone at 50 °C for 3 days.
