Table 1.
Structures of pyridinium compounds prepared for testing in the IL-8 assay
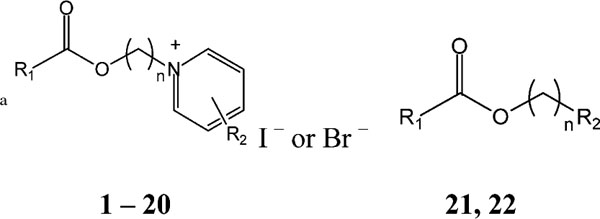
| |||
|---|---|---|---|
| R1 | Compound | n | IC50 |
| Unsubstituted pyridinium salts (R2 = H) | |||
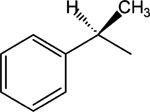
|
1 | 4 | >30 |
| 2 | 6 | >30 | |
| 3, MRS 2481 | 8 | 1.81 ± 0.58 | |
| 4 | 10 | 2.52 ± 0.39 | |
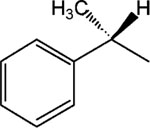
|
5, MRS 2485 | 8 | >25 |
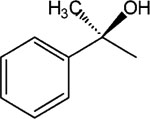
|
6 | 8 | >30 |
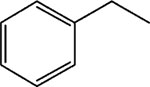
|
7 | 8 | 12 ± 0.8 |
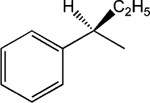
|
8 | 8 | 3.16 ± 0.52 |
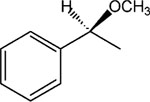
|
9 | 8 | >30 |
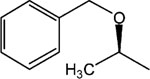
|
10 | 8 | >30 |
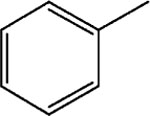
|
11 | 8 | >30 |
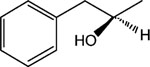
|
12 | 8 | >30 |
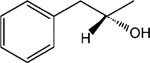
|
13 | 8 | >30 |
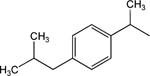
|
14 | 8 | Toxic at 1 μM |
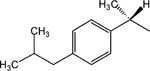
|
15 | 8 | 2.2 ± 0.8 |
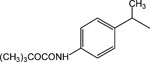
|
16 | 8 | 4.6 ± 0.9 |
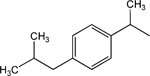
|
17 | 8 | >30 |
| R1 | Compound | R2 | IC50 |
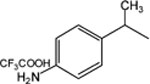
| Substituted pyridinium salts and other derivatives, n = 8 | |||
|
18 | 3-CONH2 | 5.56 ± 0.98 |
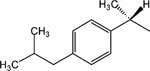
|
19 | p-(CH2)2CH3 | 3.3 ± 0.5 |
| 20 | p-(CH2)2-OH | 18 ± 0.9 | |
| 21 |
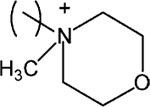
|
24 ± 1.0 | |
| 22 |
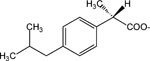
|
>30 | |
The IC50 determinations were performed in duplicate at concentrations ranging from 0.3 to 10 μM on IB-3 cells grown in 96-well microtiter plates (n = 3, or more). The solvent control containing EtOH or DMSO for initial dissolution of the compounds, which at the concentration selected minimally deviates from medium alone, is then used as a basis for 100% activity.
