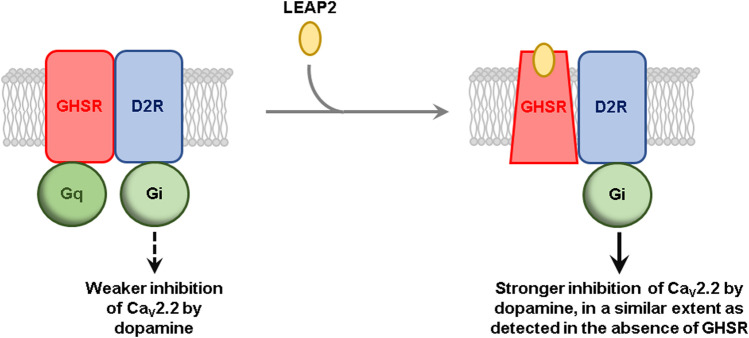
GRAPHICAL ABSTRACT.
LEAP2 Proposed model for the effect of LEAP2 on GHSR-D2R-mediated inhibition of CaV2.2. In the absence of LEAP2, GHSR is preassembled to Gq protein and in an active-like conformation due to its high constitutive activity, which impairs D2R-mediated inhibition of CaV2.2. LEAP2 stabilizes an inactive conformation of GHSR that rearranges the geometry of the GHSR-D2 heteromer and dissociates pre-assembled Gq protein, leading to a stronger inhibition of CaV2.2, in a similar extent as detected in the absence of GHSR. For the sake of simplicity, heterotrimeric G proteins are shown as a single shape.