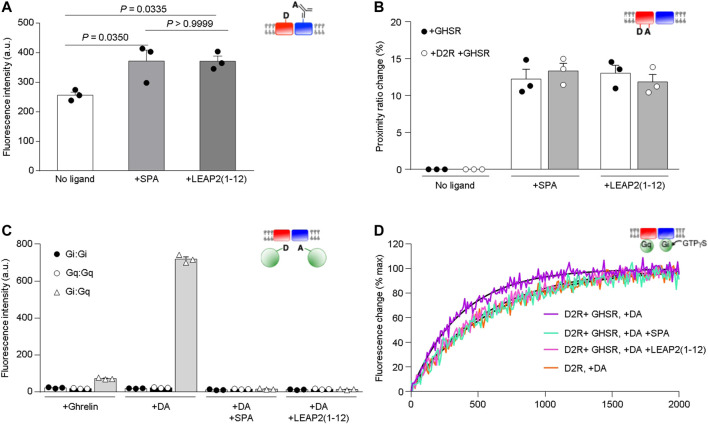
FIGURE 4.
Impact of LEAP2 on GHSR structure and dopamine-mediated Gi activation. (A) XL255 emission intensity after Tb-cryptate excitation of proteoliposomes containing Tb-cryptate labeled GHSR and XL255-labeled D2R in absence of ligand (No ligand) or in presence of 10 µM SPA (+SPA) or LEAP2 (1–12) [+LEAP2 (1–12)]. Statistical significance was evaluated by One Way ANOVA and Tukey’s post-test. (B) Proximity ratio changes induced by 10 µM of SPA (+SPA) or LEAP2 (1–12) [+LEAP2 (1–12)] calculated from the FRET signal between the fluorophores in TM1 and TM6 of GHSR assembled into lipid nanodiscs either as a homomer (+GHSR) or a heteromer (+GHSR +D2R). (C) AF-488 emission intensity after AF-350 excitation. Gαi and Gαq were labeled at their N terminus with AF-350 and AF-488, respectively, and fluorescence was measured in the presence of the labeled G proteins, the GHSR-D2R heteromer in lipid nanodiscs and 10 µM ghrelin (+Ghrelin), 10 µM dopamine (+DA), or 10 µM dopamine in the absence or in the presence of either 10 µM SPA (+DA +SPA) or LEAP2 (1–12) [+DA +LEAP2 (1–12)]. (D) GTPγS binding to Gαi1 in Gαi1β1γ2 catalyzed by the GHSR-D2R heteromer in the presence of 10 µM dopamine (DA) and in absence or in the presence of either 10 µM SPA or LEAP2 (1–12). GTPγS binding to Gαi1 catalyzed under the same conditions by the D2R homomer in the presence of 10 µM dopamine (DA) is given for comparison. The species considered are schematically depicted in all cases (red: GHSR, blue:D2R, green: G protein). Data in (A–C) is mean ± SD of three experiments.