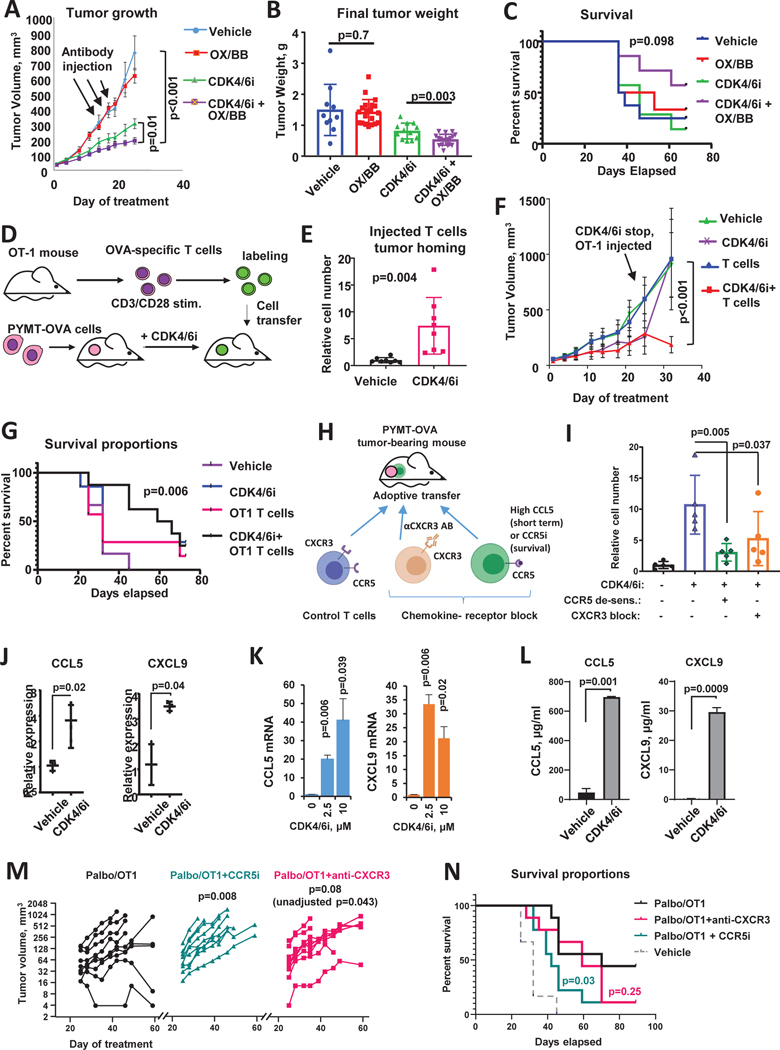
Figure 2. CDK4/6i improves immunotherapy response and promotes chemokine-mediated T cell tumor homing.
A. Tumor growth in C57Bl/6 mice implanted with OVA-expressing PYMT tumor cells and treated with CDK4/6i palbociclib (100mg/kg, wice a day), mix of agonistic antibodies for OX-40 and 4–1BB (OX/BB, 100μg each per mouse, once every 3 days), combination of both treatments, or vehicles for 25 days (n=8–10 mice, two tumors per mouse, mixed model statistics, data are represented as mean ± SEM). B. Final weight of tumors from experiment shown in A (n=10–20 tumors, one-way ANOVA, data are presented as individual values and mean ± SD). C. Survival analysis in C57Bl/6 mice implanted with OVA-expressing PYMT mammary tumor cells and treated as in A for 25 days and then followed till day 68 (n=10 mice/group, Gehan-Breslow-Wilcoxon test). D. Schematic of T cell recruitment experiment. OVA-expressing PYMT cells were implanted in 4th mammary fat pad on female C57Bl/6 mice. Mice were treated once a day with 100mg/kg palbociclib or vehicle for 2 weeks and then received IV injection of OVA-specific T cells. T cells derived from OT-1 mice were activated with anti-CD3/CD28 antibodies, and fluorescently labeled ex vivo. Tumors were collected 2 hours after cell transfer. E. Numbers of labeled T cells in tumors of C57Bl/6 mice from experiment described in D (n=8 tumors/group, t-test, data are presented as individual values and mean ± SD). F. Tumor growth in C57Bl/6 mice implanted with OVA-expressing PYMT mammary tumor cells and treated once a day with 100mg/kg CDK4/6i palbociclib or vehicle for 3 weeks followed by injection of OT-1 T cells or saline. Mice were followed until tumors reached end point size or became perforated (n=7 mice/group, mixed model, data are represented as mean ± SEM). G. Survival analysis of mice from in F. OT1 injection was given at day 25 (n=7, log-rank (Mantel-Cox) statistical comparison between Vehicle and CDK4/6i+OT1 groups is shown). Experiment was repeated with consistent results. H. Scheme of an in vivo T cell recruitment experiment. C57Bl/6 mice were inoculated with PYMT-OVA cells. Mice were treated with vehicle or CDK4/6i palbociclib at 100 mg/kg for 25 days followed by IV injection of activated OT-1 T cells. To block CCR5 and CXCR3-mediated migration, OT-1 T cells were treated with high dose of CCL5 or CXCR3-blocking antibody prior to transfer. T cells were labeled with cell tracker prior to injection into mice. I. Accumulation of fluorescent transferred T cells in PYMT tumors from experiment described in L (n=5 mice/group, one-way ANOVA, data are presented as individual values and mean ± SD). J. Real-time PCR analysis of CCL5 and CXCL10 mRNA in PYMT tumors from mice treated as in A (n=3 tumors, t-test after log transformation, data are presented as individual values and mean ± SD). K. CCL5 and CXCL9 mRNA expression in PYMT tumor cells treated with vehicle or CDK4/6i (palbociclib, 1μM) for 3 days in vitro (n=2, t-test, data are represented as mean ± SD). L. ELISA analysis of indicated chemokines in the conditioned medium of PYMT cells treated with vehicle or 1μm palbociclib for 5 days (n=2, t-test, data are represented as mean ± SD). M. Tumor volume change overtime with and without chemokine receptor blockade. Female C57Bl/6 mice were implanted with PYMT-OVA tumors and treated with palbociclib for 25 days and injected with activated OT-1 T cells (treatment scheduler was same as in F). A subgroup of mice received CCR5i maraviroc (10 mg/kg, once daily oral gavage) or CXCR3-blocking antibody (200 μM per mouse, IV injection every 3 days) on days 25–42. Mixed model with Dunnet’s post-test was used for statistical comparison of tumor growth between control CDK4/6i+OT-1 group and chemokine-receptor-inhibited groups (n=9 mice per group, individual data presented). p-value with and without adjustment for multiple comparison is sown for CXCR3-blockade group. N. Survival curves from experiment in M (n=9, log-rank (Mantel-Cox) statistical comparison between CDK4/6i+OT1 and chemokine-receptor inhibited groups).