Fig. 1 |. Spinal cord injury: pathophysiological events and potential therapeutic targets.
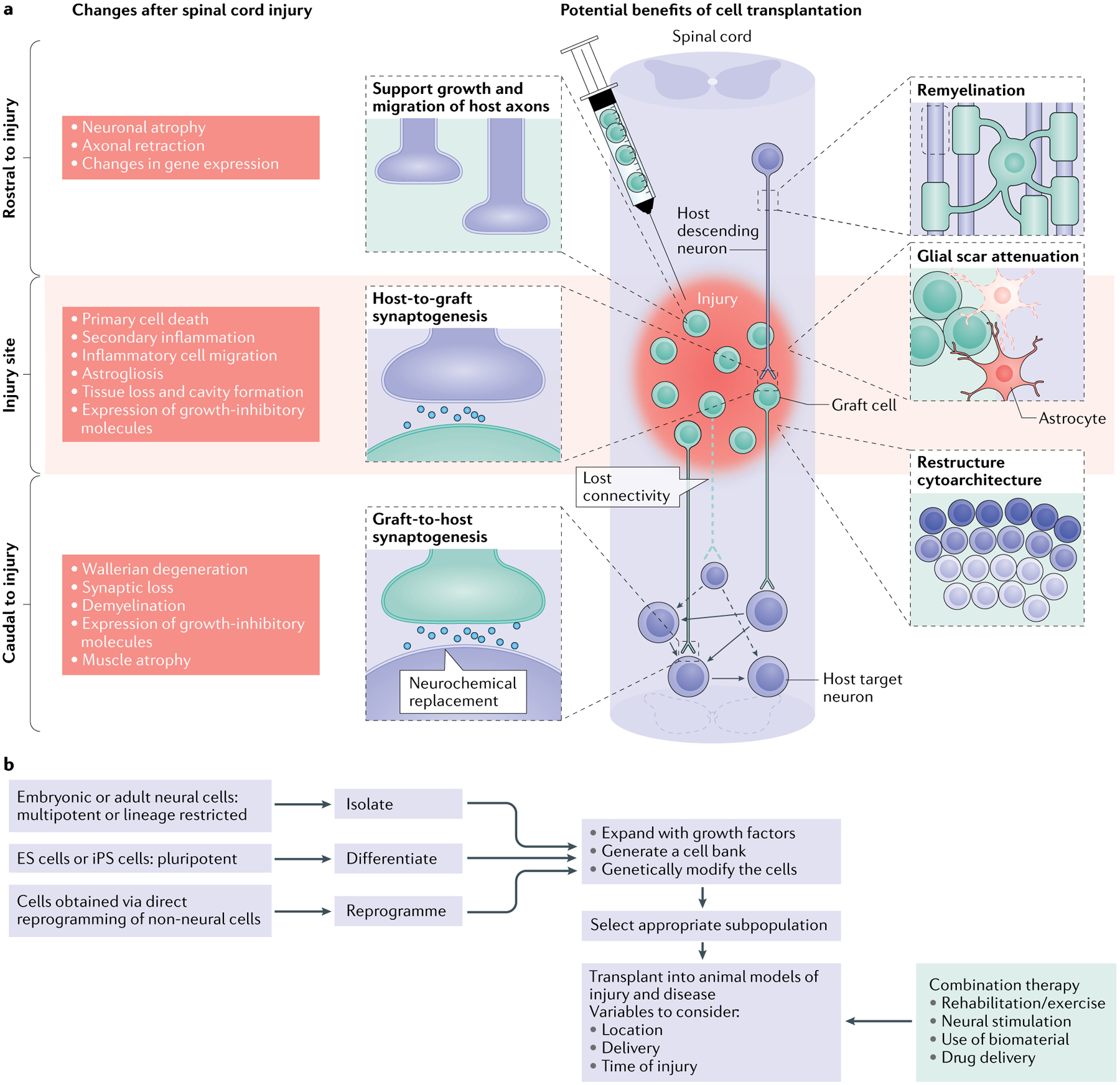
a | The left side of the schematic illustrates the complex changes that occur after spinal cord injury (SCI), which differ temporally and spatially. In a descending tract, such as that illustrated here, these changes include events rostral to the injury (including axon degeneration and changes in gene expression), at the level of the injury (encompassing acute tissue damage and cell death as well as chronic secondary injury and inflammation) and caudal to the injury (including both neural events such as demyelination and non-neural events such as muscle atrophy). Similar changes occur in ascending tracts; however, in this case the location of the events in relation to the injury will be reversed. The injured spinal cord schematic illustrates potential therapeutic targets for cell transplantation, including remyelination, support of host axon growth, glial scar attenuation, synaptogenesis and the restructuring of spinal cord cytoarchitecture. b | The flow chart depicts the decisions that must be made when a cell transplantation strategy for SCI is being developed and the processing steps involved. It shows choices of cells for transplantation in SCI, the process of their preparation, modification and selection and the parameters of their delivery alone and as part of a combination therapy. For cell choices, a wide range of neural progenitor cells can be obtained from embryonic and adult tissue, from pluripotent cells (embryonic stem cells (ES cells) and induced pluripotent stem cells (iPS cells)) and from direct reprogramming of non-neural cells. These cells can be expanded with growth factors to generate cell banks and/or can be genetically modified (to overexpress growth factors, for example). It is then possible to select a subpopulation of the resulting neurons for transplantation. The transplantation process needs to consider variables such as the location of the transplant (that is, transplantation directly into the injury site, intrathecally or systemically), the delivery method (that is, injection as a cell suspension or as part of a hydrogel scaffold) and the timing (for example, subacute transplantation versus transplantation after a 2-week delay after injury). A number of different types of combination therapy can also be initiated at different times and act synergistically with the transplant. In particular, attention should be paid to rehabilitation strategies, various neural stimulation modalities, the use of biomaterials and drug delivery.
