Fig. 3 |. Restoring connectivity in the respiratory system.
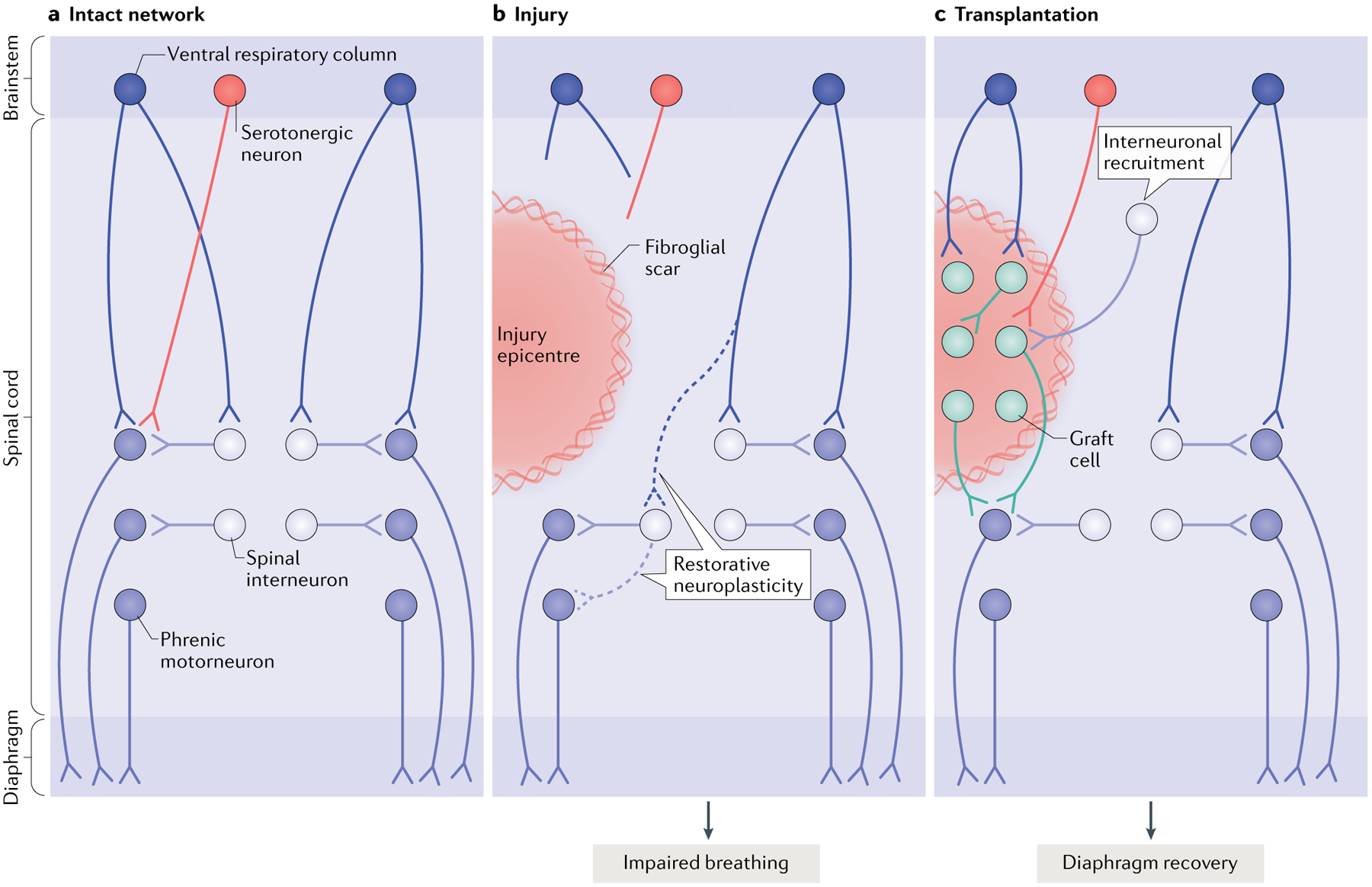
The diagram depicts the intact (part a), injured (part b) and transplant-treated (part c) spinal phrenic motor circuit within the cervical spinal cord. Respiration is driven by brainstem neurons in the ventral respiratory column that directly — or indirectly via spinal interneurons — innervate the phrenic motor neuron pool (distributed from cervical level C3 to cervical level C6). Phrenic motor neuron activity is also modulated by serotonergic pathways and populations of spinal interneurons as breathing conditions change. Phrenic motor neurons on each side of the spinal cord innervate half of the diaphragm on each side of the body via phrenic nerves. Injury (part b) can compromise descending projections, as well as phrenic spinal interneurons and motor neurons. Spared spinal neurons caudal to the injury are therefore denervated. While this is devastating, some limited recovery of diaphragm activity can occur ipsilateral to the injury via restorative neuroplasticity (dashed lines), and spared monosynaptic and polysynaptic pathways from the contralateral spinal cord (via brainstem and spinal interneurons, respectively) can facilitate plasticity in these lateralized spinal injuries. However, the extent of recovery is minimal and deficits persist. A number of cell therapies have been used to promote repair and plasticity within injured respiratory pathways. Neural progenitor cell transplants are perhaps the most often used, as they can modify glial scarring at the lesion border and provide the building blocks for tissue repair. Transplantation of neural progenitor cells into the injured phrenic network in animal models (typically directly into the lesion site as shown in part c) has resulted in extensive synaptic integration between donor neurons themselves, between host spinal and brainstem neurons and donor neurons and between donor and spinal phrenic neurons. This synaptic integration also coincides with enhanced plasticity of existing and newly formed pathways, and improved respiratory activity15,18,22,23. Without any other intervention, transplantation of cells alone is likely to lead to the formation of a vast range of new connections, which are likely to differ between treated recipients22,23, and to the recruitment of novel interneuron populations to establish novel neural networks. Research is under way to develop strategies that control this integration and connectivity. Other models of injury affecting the phrenic network and additional mechanisms of recovery are discussed elsewhere239.
