FIG 2.
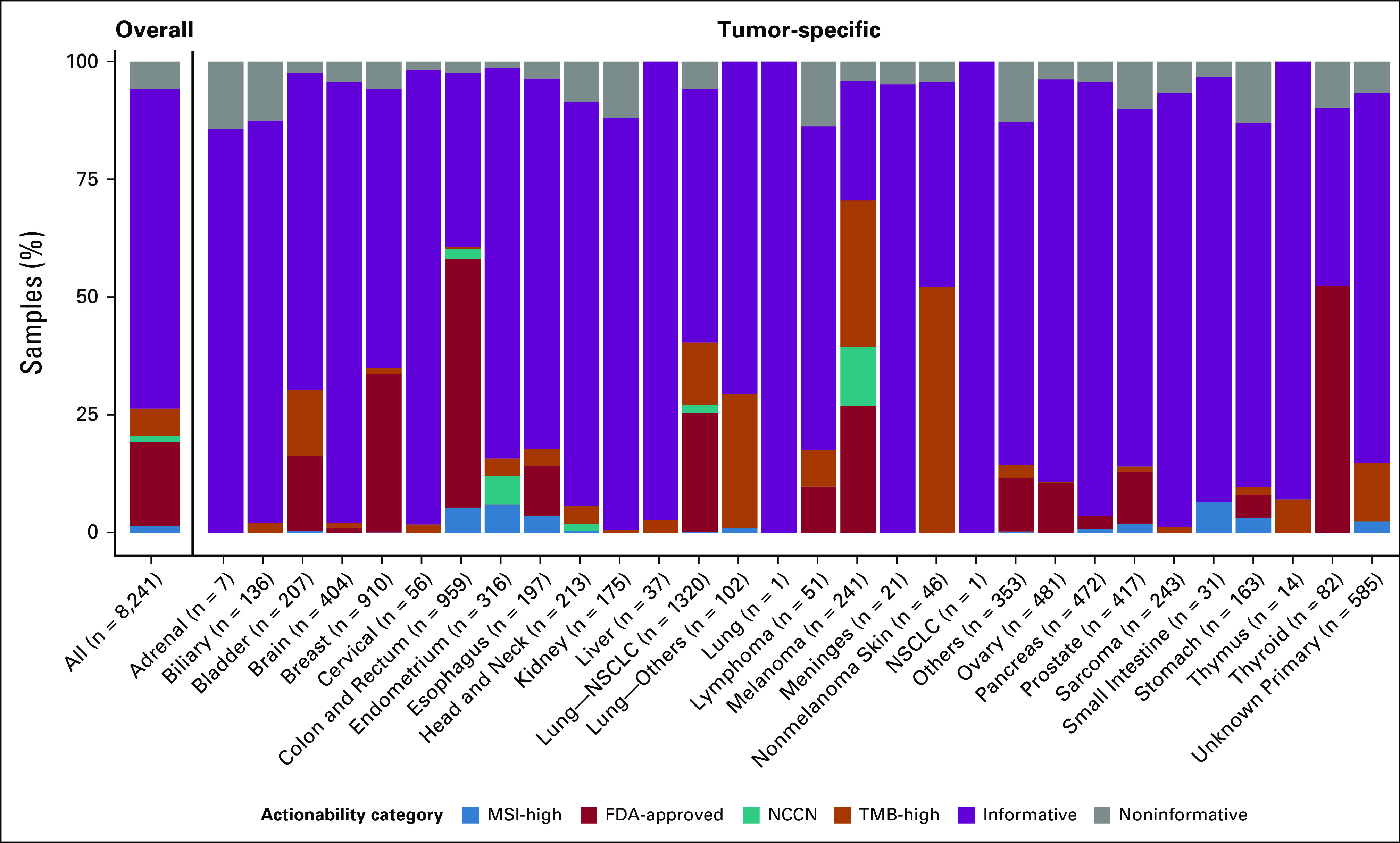
Pan-cancer assessment of potential actionability from PCR-CGP testing. All sample QC input-evaluable samples profiled between January 1 and June 25, 2020 (n = 8,241), were stratified by tumor type and assigned to one actionability class on the basis of MSI-H status, presence of an FDA-approved (within cancer type) biomarker, presence of an NCCN guideline–recommended (within cancer type) biomarker, and other TMB-H (≥ 10 mutations/megabase as TMB-H) using the associated therapy logic used in current StrataNGS reporting. Samples without one of these biomarkers were considered informative if at least one prioritized biomarker was reported or the sample passed all sequencing QC metrics with ≥ 20% TC. All other samples were considered test failures. CGP, comprehensive genomic profiling; FDA, US Food and Drug Administration; MSI-H, microsatellite instability–high; NCCN, National Comprehensive Cancer Network; NSCLC, non–small-cell lung cancer; PCR-CGP, multiplex polymerase chain reaction–based comprehensive genomic profiling; QC, quality control; TC, tumor content; TMB, tumor mutation burden; TSA, tumor surface area.
