Abstract
Extragonadal non-gestational choriocarcinoma is a rare but well-described phenomenon occurring in patients with midline germ cell tumors. Choriocarcinoma (ChC) is an aggressive neoplasm usually developing in women as a rare complication of pregnancy. In male patients ChC occurs in the testes, usually as a component of mixed germ cell tumors. Very few patients develop extragonadal choriocarcinoma with the tumor occurring in midline locations, such as the mediastinum, retroperitoneum, and central nervous system (mostly pineal gland). Non-midline choriocarcinoma can occur in the lung, gastrointestinal tract, and breast, sometimes blended with another primary malignancy. A midline choriocarcinoma manifesting as a head and neck malignancy is exceptional. During an evaluation of multiple enlarged cervical lymph nodes suspected to be lymphoma in a 72-year-old man, a core biopsy was taken from one of the left neck lymph nodes which histologically showed a necrotic malignancy with strong diffuse pancytokeratin staining. After an initial interpretation of metastatic carcinoma, further samples were taken from both tonsils and from a right level 5 neck lymph node. Histologically, all samples contained the same tumor, showing profound pleomorphism and multinucleated syncytial-type giant cells. A panel of immunohistochemistry studies were performed, including β-human chorionic gonadotropin, with positive findings leading to a diagnosis of extragonadal non-gestational choriocarcinoma.
Keywords: Extragonadal non-trophoblastic choriocarcinoma, Midline, Metastases, Palatine tonsil, Male, β-Human chorionic gonadotropin, Immunohistochemistry
Introduction
Male extragonadal non-gestational choriocarcinoma (ChC) is a rare but well-described phenomenon occurring in patients with midline germ cell tumors. Previous literature has reported that mediastinum, central nervous system, and retroperitoneum are the most common sites of extra-gonadal ChC, with the pineal region the most common CNS site [1–4], whereas lung, breast, and gastrointestinal tract are the most prevalent organ locations beyond midline sites [5, 6].
Metastases to the oral cavity or oropharynx are rare. The most common tumor sites of origin are lungs (20%), kidney (16%), breast (11%), and gastrointestinal tract (colon and stomach (9%) [7, 8], with only a few cases reported in the English literature of ChC metastatic to the oral cavity but not oropharynx [8–14]. Extragonadal non-gestational midline ChC is a malignant, germ cell tumor composed of extraembryonic chorion that includes cytotrophoblastic and syncytiotrophoblastic cells, representing an incidence of 0.0022 per 1,000,000 people [6]. There are a few case reports of extragonadal midline ChC metastasizing to cervical lymph nodes [15], but to date, an oropharyngeal extragonadal non-gestational ChC has not been reported.
It is important to recognize extragonadal ChC can occur in the head and neck region outside of the CNS system, and immunohistochemical confirmation is essential to reach the correct diagnosis. We herein report a case of ChC manifesting initially as bilateral tonsillar masses with bilateral neck lymph node involvement, with subsequent midline mediastinal disease identified. Additionally, we discuss the diagnostic challenges posed by midline germ cell tumors and their separation from other primary and metastatic carcinomas. The unusual morphologic findings of giant cells coupled with the immunohistochemistry results allow for recognition of this hitherto unreported type of oropharyngeal malignancy.
Case Report
Clinical Presentation
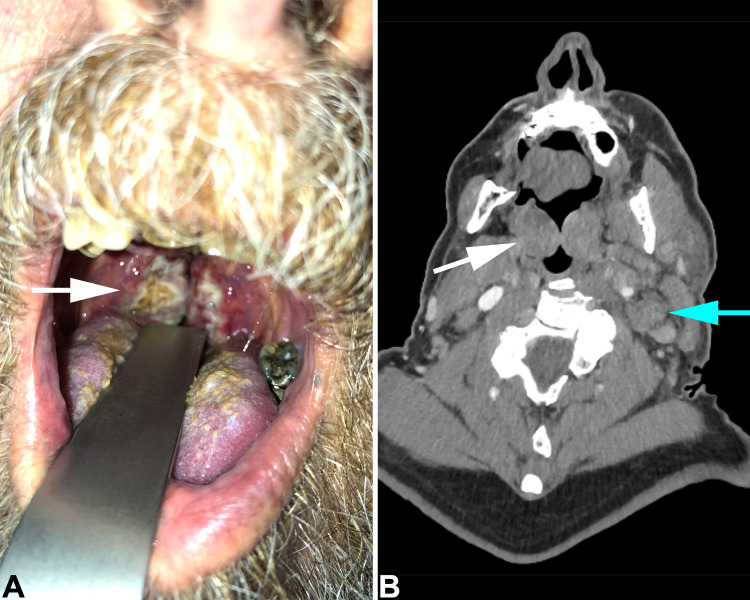
A 72-year-old man presented with a 2 week history of weight loss and rapidly progressive cervical lymphadenopathy, causing dysphagia and dyspnea. Physical assessment showed grade 4 bilateral tonsillar enlargement (his tonsils occupied > 75% of the oropharyngeal width) [16] (Fig. 1a). An urgent CT scan was requested (Fig. 1b). His previous medical history included asthma, hypertension, and atrial fibrillation. He had no previous surgery and a genital examination was unremarkable. Based on his clinical findings, a working diagnosis of lymphoma was used to obtain an urgent core needle biopsy from one of the enlarged left cervical lymph nodes.
Fig. 1.
a Intraoral view of palatine tonsils. Both tonsils are enlarged and covered by necrotic material (white arrow). b Coronal CT scan shows enlarged palatine tonsils (white arrow) and large left neck lymph nodes (blue arrow)
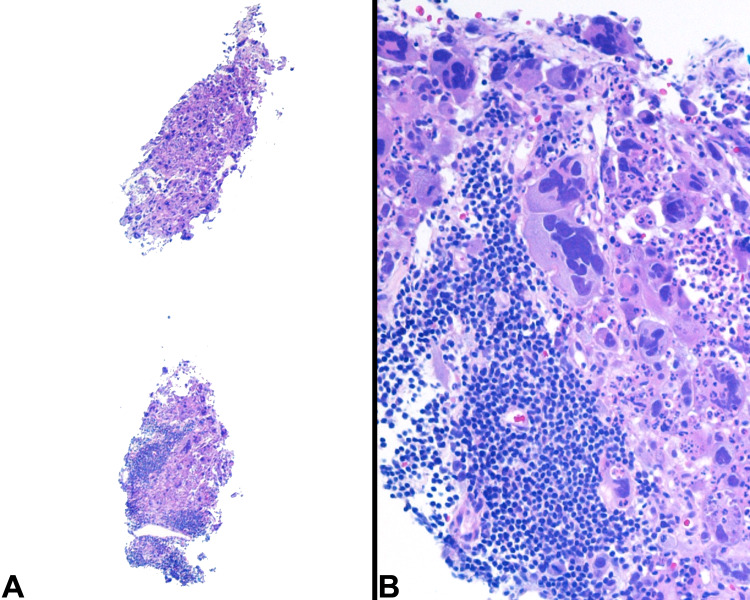
With mostly necrotic tumor (Fig. 2), a histologic diagnosis of poorly differentiated carcinoma metastatic to a lymph node was rendered, with the patient referred to the ear, nose, and throat (ENT) department for further assessment of a presumed primary head and neck malignancy. The cervical lymph nodes rapidly increased in size, with concurrent weight loss. A biopsy of the tonsils (Fig. 3) was performed along with a biopsy of the right neck level V lymph node mass under local anesthetic.
Fig. 2.
a Core biopsy from left neck lymph node shows highly necrotic malignant tumor. The cells have pleomorphic nuclei, prominent nucleoli with some cell containing multiple nuclei. b The neoplastic cells show a strong and diffuse cytoplasmic reactivity for pancytokeratin (AE1/AE3) by immunohistochemistry while c non-reactive for CD45RB which only highlighted lymphocytes
Fig. 3.
a Right tonsil incisional biopsy shows replacement by a highly cellular neoplastic proliferation mostly undermining the squamous mucosa, but also showing pagetoid spread. There are cells with multinucleation. b The neoplastic cells show a strong and diffuse pancytokeratin reaction, including highlight pagetoid cells. c β-hCG stains all tumor cells. d β-hCG is seen in the cytoplasm of both cyto- and syncytiotrophoblastic-like cells. e There is no tumor reactivity for AFP. f There is a strong nuclear reaction with MIB1, yielding a proliferation index of about 70%
Pathology
The left neck lymph node ultrasound-guided core needle biopsy performed at initial presentation consisted of five necrotic and hemorrhagic cores ranging from 5 to 10 mm in length. They were almost entirely composed of tumor with a limited amount of lymphoid tissue, lacking well developed lymph node architecture. The tumor was arranged in a syncytial pattern with poor cohesion. Some neoplastic cells were spindled, others had eccentric nuclei with dense and eosinophilic cytoplasm, others had multilobulated nuclei with prominent nucleoli and there were scattered multinucleated giant cells (Fig. 2a). The tumor cells had abundant cytoplasm, profound nuclear pleomorphism, and an average of 9 atypical mitoses/2 mm2 were seen. A panel of immunohistochemical studies were performed, with the neoplastic cells positive for cytokeratin AE1/AE3, CAM5.2 (Fig. 2b), MNF116, 34βE12, EMA, and CK7, while negative for CD45RB (Fig. 2c), CD20, CD3, CD30, CD5, CD4, CD15, SOX10, p63, and p16. In situ hybridization for EBER was negative. A diagnosis of metastatic poorly differentiated carcinoma was rendered. While there was less necrosis in the right level V lymph node sample, the histologic features of the core biopsy were identical (Fig. 4).
Fig. 4.
a Low power view of core biopsy from right posterior neck node. b The cores show tumor with pronounced nuclear pleomorphism and multinucleation
Biopsies from both tonsils showed tumor, with a small portion of unremarkable squamous mucosa in the left tonsil only (Fig. 3a) showing a few highly atypical cells within the epithelium in a Pagetoid pattern, highlighted by CAM5.2 positive immunostaining (Fig. 3b). The bulk of the tumor was beneath the squamous mucosa. The histologic features were similar to those seen in the right and left core needle biopsies from the neck lymph nodes. The tonsillar tumor lacked pigmentation, keratinization, and well-developed intercellular borders. However, the multinucleated tumor giant cells showed a deeply eosinophilic cytoplasm surrounding multiple dark and smudged nuclei. The multinucleated cells were quite reminiscent of syncytiotrophoblasts. Immunohistochemical stains were positive for cytokeratins AE1/AE3 and CAM5.2 (Fig. 3b), with strong cytoplasmic staining for β-human chorionic gonadotropin (β-hCG) (Fig. 3c, d). Staining for AFP and PLAP was negative (Fig. 3e). MIB-1 immunohistochemistry was present in up to 70% of the viable tumor cells (Fig. 3f). These findings supported a diagnosis of extragonadal (non-gestational) ChC involving both tonsils and bilateral neck lymph nodes. Further clinical workup documented elevated serum β-hCG of 1502 IU/L (reference range, 0–4 IU/L for adult male). Imaging studies (computed tomography of the neck, thorax, abdomen, and pelvis) documented additional lymphadenopathy, including bulky mediastinal lymphadenopathy, bilateral axillary, and inguinal lymph nodes. His genital examination was normal without testicular mass (confirmed by ultrasound).
Discussion
The rare phenomenon of extragonadal, non-trophoblastic ChC arising in the midline without a testicular primary was first reported in the German literature by Davidson et al. [17]. Since that description, most additional articles have been single case reports or small series, no doubt related to its rarity. A total of 115 cases of extragonadal ChC in male patients between 1973 and 2017 were reviewed by Qiu et al. [6]. The overall incidence rate was 0.0022 per 1,000,000 people with an age range from 0 to 84 years of age. The most common tumor location was along the midline, such as the mediastinum, retroperitoneum, and pineal gland [6]. Equally rare is the phenomenon of choriocarcinomatous differentiation of tumor cells in otherwise typical carcinoma of various organs. Without midline distribution, ChC is reported in the stomach (53 patients) [18] and colon (12 cases). When reported in the colon, some are associated with concurrent adenocarcinoma [6], some arising within pre-existing colitis [19], and others within otherwise histologically normal colon [20]. Monn et al. [21] reported a single case arising in the bladder. Breast cancer has been reported with a choriocarcinomatous component [22] with another case arising without a concurrent breast ductal/lobular primary carcinoma [23]. Despite a comprehensive search of the English literature, there are no primary cases of oropharyngeal ChC. The ChC involving the gingiva (oral cavity rather than oropharynx) reported by Sato et al. [24] in 1978 arose in a female with a previous hydatidiform mole, and is thus, gestational and metastatic. Another case reported a 25-year-old male with testicular cancer and an epulis-like metastasis to the buccal mucosa [25]. Exceptionally, extragonadal germ cell tumors can occur in the head and neck area outside of the central nervous system in pediatric patients. The tumor is reported in a teratoma [26] or even as a malignant component of a teratocarcinosarcoma [27]. Roy et al. [28] reported two cases of cheek (buccal) yolk sac tumor with strong positive staining of the neoplastic cells for AFP in male children, aged 1 year 7 months and 3 years 5 months. It is possible a mediastinal primary accounted for the patient’s disease in this case report, but it would still represent extragonadal midline disease, although impossible to determine definitively.
Diagnosis of limited, small biopsies of oropharyngeal (base of tongue, tonsils, adenoids) lesions can be challenging. HPV-associated and HPV-independent squamous cell carcinoma are by far the most common primary malignancies [29], but rare primary and metastatic tumors (such as benign or malignant teratoma, teratocarcinosarcoma, yolk sac tumor, follicular dendritic cell sarcoma, synovial sarcoma, melanoma, various salivary gland primaries, and metastatic tumors) can occur at this site, substantially expanding the differential diagnosis [26–28, 30].
In this reported case, the diagnostic dilemma is between metastatic ChC to both tonsils and primary extra-gonadal ChC with synchronous, midline and symmetrical, distribution in both tonsils, bilateral cervical and axillary lymph nodes, and mediastinum. The diagnosis of extra-gonadal ChC requires the patient to have: (a) no previous testicular malignancy; (b) elevated serum β-hCG; and (c) radiological evidence of a midline tumor. As all of these features were found concurrently rather than sequentially, the preponderance of evidence suggests a synchronous midline and symmetrical distribution of primary extra-gonadal non-gestational ChC. It is important to recognize tonsillar disease as midline and potentially part of multicentric or metastatic disease.
ChC is a highly aggressive germ cell malignancy. It usually occurs in females as a complication of gestation. In males, ChC occurs in the testes, usually as a component of a mixed testicular germ cell tumor, or rarely as a pure form. It is rare in childhood, unless the patients have disordered sex development [31] and otherwise represents ~ 1% of all male malignancies occurring in reproductive age [32]. Midline tumors in males are classified as extragonadal. Preferential sites are the mediastinum, retroperitoneum, and CNS, with symmetrical involvement of lymph nodes. The most accepted hypothesis of extragonadal ChC is its origin from retained primordial germ cells during their migration to the gonads, although other theories suggest they represent metastasis from a subsequently regressed gonadal ChC or multiphenotypic differentiation from undifferentiated stem cells. Invariably, male extragonadal ChC demonstrates elevated levels of β-hCG. The histological diagnosis of extragonadal ChC is difficult, often requiring more than one biopsy and consequent delay in diagnosis [33]. If a pertinent panel of immunohistochemistries is performed, specifically including a pancytokeratin and β-hCG, then the diagnosis is more easily reached. An initial panel approach to include markers such as pancytokeratin, p63/p40, synaptophysin, S100 protein, SOX10, CD99, desmin, and CD45 are helpful in further narrowing the focus of what other additional studies may be of value. This panel allows for consideration of several epithelial tumors, neuroendocrine neoplasms, melanoma, sarcoma, and lymphoma. In the exceptionally unique case presented, the presence of a positive reaction to pancytokeratin and p63, would make a primary epithelial tumor the top potential consideration. However, multinucleated, syncytial-like giant cells along with a heavily necrotic background tumor are not common in primary tumors, although tumor giant cells are seen in a fair proportion of oropharyngeal squamous cell carcinomas [34]. In general, patients with gonadal ChC are usually in their reproductive age with a testicular tumor. Without the original panel showing a poorly differentiated carcinoma rather than lymphoma, one would be hard pressed to consider β-hCG in a 72-year-old male with normal testes and bilateral tonsil and neck lymph node enlargement. It was only because of the unusual tumor giant cells and extensive necrosis that additional immunohistochemistry studies for β-hCG, AFP, and PLAP aided in reaching the correct diagnosis.
ChC is known for rapid growth, vascular invasion, and a tendency to outgrow its blood supply with extensive tumor necrosis, findings seen on the current biopsies [32]. Nodal and hematogenous metastases, especially to the lung, are common complications.
Despite the rarity of ChC in males, and even more so for extragonadal location, special attention must be given to the patient’s clinical presentation to ensure an accurate diagnosis is rendered. A pathologist’s index of suspicion should be high for tumors with unusual morphology or inconsistent with the typical presentation of head and neck primary malignancies.
In this case, the patient received chemotherapy-based treatment, however, due to the rapid progression of this aggressive malignancy, he succumbed to his disease within two weeks of starting treatment.
Acknowledgements
The authors wish to thank Dr. Kate Potter for her contribution with radiological imaging.
Funding
No external funding was obtained for this study.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest as it relates to this research project.
Ethical Approval
All procedures performed in this retrospective data analysis involving human participants were in accordance with the ethical standards of the institutional review board (IRB #5968). Patient consent was obtained for a case report and clinical photography (available on request). The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Matsutani M, Sano K, Takakura K, Fujimaki T, Nakamura O, Funata N, et al. Primary intracranial germ cell tumors: a clinical analysis of 153 histologically verified cases. J Neurosurg. 1997;86(3):446. doi: 10.3171/jns.1997.86.3.0446. [DOI] [PubMed] [Google Scholar]
- 2.Shinoda J, Sakai N, Yano H, Hattori T, Ohkuma A, Sakaguchi H. Prognostic factors and therapeutic problems of primary intracranial choriocarcinoma/germ-cell tumors with high levels of HCG. J Neurooncol. 2004;66(1–2):225–240. doi: 10.1023/B:NEON.0000013499.74404.81. [DOI] [PubMed] [Google Scholar]
- 3.Jiang T, Raynald YH, Zhang W, Li C. Predictive factors of overall survival in primary intracranial pure choriocarcinoma. J Clin Neurosci. 2019;61:93–101. doi: 10.1016/j.jocn.2018.10.136. [DOI] [PubMed] [Google Scholar]
- 4.Kurita T, Iwasa K, Yano H. Primary extragenital choriocarcinoma in spinal cord: report of a case. Hinyokika Kiyo. 1966;12(5):466–470. [PubMed] [Google Scholar]
- 5.Schmoll HJ. Extragonadal germ cell tumors. Ann Oncol. 2002;13(Suppl 4):265–272. doi: 10.1093/annonc/mdf669. [DOI] [PubMed] [Google Scholar]
- 6.Qiu J, Jia S, Li G. Incidence and prognosis factors of extragonadal choriocarcinoma in males: a population-based study. Cancer Manag Res. 2018;10:4565–4573. doi: 10.2147/CMAR.S175948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Owosho AA, Xu B, Kadempour A, Yom SK, Randazzo J, Ghossein RA, et al. Metastatic solid tumors to the jaw and oral soft tissue: a retrospective clinical analysis of 44 patients from a single institution. J Cranio-Maxillofac Surg. 2016;44(8):1047–1053. doi: 10.1016/j.jcms.2016.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimura Y, Yakata H, Kawasaki T, Nakajima T. Metastatic tumours of the mouth and jaws. A review of the Japanese literature. J Maxillofac Surg. 1982;10(4):253–258. doi: 10.1016/S0301-0503(82)80050-7. [DOI] [PubMed] [Google Scholar]
- 9.Gomes JB, Santos PS, Felix VB, Prospero JD, Nunes CC, de Freitas RR. Oral lesion as the first manifestation of choriocarcinoma of the testicle. J Clin Oncol. 2009;27(9):1522–1523. doi: 10.1200/JCO.2008.20.2226. [DOI] [PubMed] [Google Scholar]
- 10.Bhatia K, Vaid AK, Rawal S, Patole KD. Pure choriocarcinoma of testis with rare gingival and skin metastases. Singapore Med J. 2007;48(3):e77–80. [PubMed] [Google Scholar]
- 11.Englert RJ, Pasqual HN. Metastatic chorionepithelioma of the gingival tissue; report of a case. Oral Surg Oral Med Oral Pathol. 1957;10(8):813–818. doi: 10.1016/0030-4220(57)90109-3. [DOI] [PubMed] [Google Scholar]
- 12.Catania AF. Three nonvital teeth associated with chorio-epithelioma: report of a case. J Oral Surg. 1953;11(4):324–330. [PubMed] [Google Scholar]
- 13.Bakeen G, Hiyarat AM, Al-Ubaidy SS. Chorioepithelioma presenting as a bleeding gingival mass. Oral Surg Oral Med Oral Pathol. 1976;41(4):467–471. doi: 10.1016/0030-4220(76)90274-7. [DOI] [PubMed] [Google Scholar]
- 14.Yenen E, Dirvana S, Babuna C. Ectopic choriocarcinoma. Report of a case with unusual location. Obstet Gynecol. 1967;30(4):556–559. [PubMed] [Google Scholar]
- 15.Şenkal HA, Yilmaz T, Sözeri AB. Metastatic choriocarcinoma: a rare presentation as a neck mass. Ear Nose Throat J. 2013;92(6):E42–E46. doi: 10.1177/014556131309200622. [DOI] [PubMed] [Google Scholar]
- 16.Brodsky L. Modern assessment of tonsils and adenoids. Pediatr Clin N Am. 1989;36(6):1551–1569. doi: 10.1016/S0031-3955(16)36806-7. [DOI] [PubMed] [Google Scholar]
- 17.Davidson C. Chorionepitherion und Magenkrebs, eine Seltene Vershmelzung zweierbosartiger Geschwulste. Carite Ann. 1905;29:426–437. [Google Scholar]
- 18.Kobayashi A, Hasebe T, Endo Y, Sasaki S, Konishi M, Sugito M, et al. Primary gastric choriocarcinoma: two case reports and a pooled analysis of 53 cases. Gastric Cancer. 2005;8(3):178–185. doi: 10.1007/s10120-005-0332-9. [DOI] [PubMed] [Google Scholar]
- 19.Pezzuto F, Fortarezza F, Falcone V, Quintiliani C, Piscitelli D. Primary intestinal choriocarcinoma in a patient with long-standing Crohn's disease. G Chir. 2017;38(3):147–148. doi: 10.11138/gchir/2017.38.3.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang L, Wu J-T, Peng X. Primary choriocarcinoma of the colon: a case report and review of the literature. World J Surg Oncol. 2013;11(1):23. doi: 10.1186/1477-7819-11-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Monn MF, Jaqua KR, Bihrle R, Cheng L. Primary choriocarcinoma of the bladder: a case report and review of literature. Clin Genitourin Cancer. 2017;15(2):188–191. doi: 10.1016/j.clgc.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 22.Saigo PE, Rosen PP. Mammary carcinoma with “choriocarcinomatous” features. Am J Surg Pathol. 1981;5(8):773–778. doi: 10.1097/00000478-198112000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Y, Liu M, Li J, Jing F, Linghu R, Guo X, et al. Breast carcinoma with choriocarcinomatous features: a case report and review of the literature. World J Surg Oncol. 2014;12:239. doi: 10.1186/1477-7819-12-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sato M, Nishio J, Yoshida H, Maeda N, Urade M, Miyazaki T, et al. Metastatic choriocarcinoma involving the gingiva. Int J Oral Surg. 1978;7(3):192–196. doi: 10.1016/S0300-9785(78)80024-6. [DOI] [PubMed] [Google Scholar]
- 25.Lee L, Oppenheimer R, Jayaram L. Germ cell tumor metastatic to the oral cavity. Ear Nose Throat J. 2012;91(4):172–173. doi: 10.1177/014556131209100410. [DOI] [PubMed] [Google Scholar]
- 26.Brodsky JR, Irace AL, Didas A, Watters K, Estroff JA, Barnewolt CE, et al. Teratoma of the neonatal head and neck: a 41-year experience. Int J Pediatr Otorhinolaryngol. 2017;97:66–71. doi: 10.1016/j.ijporl.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 27.Rotenberg B, El-Hakim H, Lodha A, MacCormick A, Ngan BY, Forte V. Nasopharyngeal teratocarcinosarcoma. Int J Pediatr Otorhinolaryngol. 2002;62(2):159–164. doi: 10.1016/S0165-5876(01)00575-4. [DOI] [PubMed] [Google Scholar]
- 28.Roy M, Agarwal S, Gupta A, Bakhshi S, Bhalla A. Extragonadal yolk sac tumor of the head and neck region: a report of two cases. J Cancer Res Therap. 2015;11(4):1000–1002. doi: 10.4103/0973-1482.157305. [DOI] [PubMed] [Google Scholar]
- 29.Chaturvedi AK, Anderson WF, Lortet-Tieulent J, Curado MP, Ferlay J, Franceschi S, et al. Worldwide trends in incidence rates for oral cavity and oropharyngeal cancers. J Clin Oncol. 2013;31(36):4550–4559. doi: 10.1200/JCO.2013.50.3870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Osorio M, Moubayed SP, Hernandez-Prera J, Scott JC, Urken ML. Primary mucosal melanoma of the palatine tonsil: Report of a case and review of the literature. Am J Otolaryngol. 2017;38(4):501–504. doi: 10.1016/j.amjoto.2017.04.010. [DOI] [PubMed] [Google Scholar]
- 31.Krag Jacobsen G, Barlebo H, Olsen J, Schultz HP, Starklint H, Søgaard H, et al. Testicular germ cell tumours in Denmark 1976–1980. Pathology of 1058 consecutive cases. Acta Radiol Oncol. 1984;23(4):239–247. doi: 10.3109/02841868409136019. [DOI] [PubMed] [Google Scholar]
- 32.Rejlekova K, Cursano MC, De Giorgi U, Mego M. Severe complications in testicular germ cell tumors: the choriocarcinoma syndrome. Front Endocrinol. 2019;10:218. doi: 10.3389/fendo.2019.00218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qiu Z, Wu Y, Wang Y, Hu C. Male primary mediastinal choriocarcinoma with diffuse metastases: a case report. Medicine. 2019;98(28):e16411. doi: 10.1097/MD.0000000000016411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson LDR, Burchette R, Iganej S, Bhattasali O. Oropharyngeal squamous cell carcinoma in 390 patients: analysis of clinical and histological criteria which significantly impact outcome. Head Neck Pathol. 2020;14(3):666–688. doi: 10.1007/s12105-019-01096-0. [DOI] [PMC free article] [PubMed] [Google Scholar]