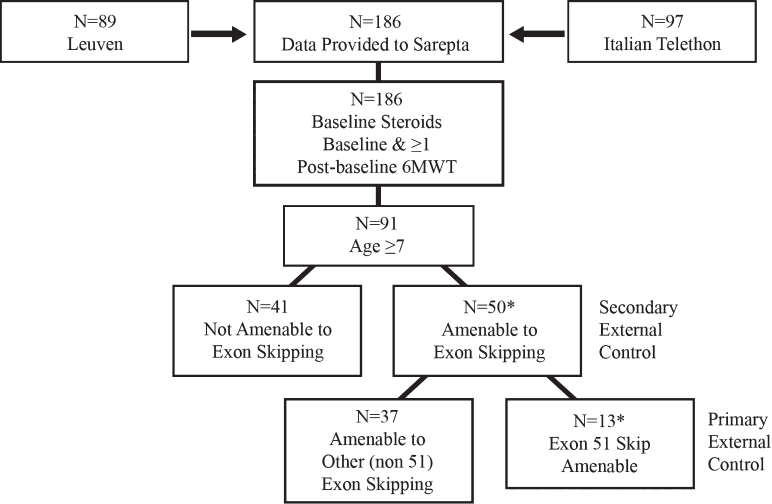
Fig. 2.
Primary external controls for ambulatory assessment were derived from patient-level natural history data from Duchenne muscular dystrophy centers of excellence participating in the Italian Telethon and Leuven registries.* *2 patients were excluded from the analysis for the following reasons: •Patient OBG 16 withdrew consent. •Though included in earlier reports, it was later determined that Patient OBG 20 was enrolled in a drisapersen trial in Year 2 and lost ambulation while on drisapersen.