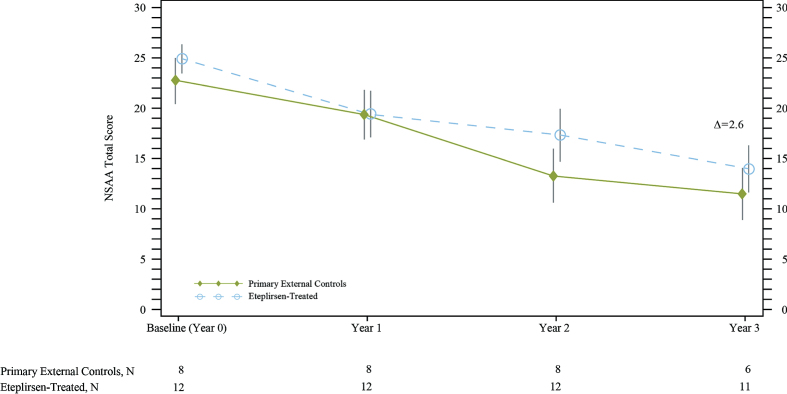
Fig. 5.
North Star Ambulatory Assessment (NSAA) total score showed that eteplirsen-treated patients experienced less decline compared with primary external controls over 3 years. Δ represents the unadjusted difference in mean change from baseline in eteplirsen-treated patients versus external controls. NSAA data for primary external controls were available from the Italian Telethon registry patients only (8 of the 12 natural history patients used for ambulatory comparisons). In the eteplirsen-treated group, 1 patient did not have NSAA measured at Year 3.