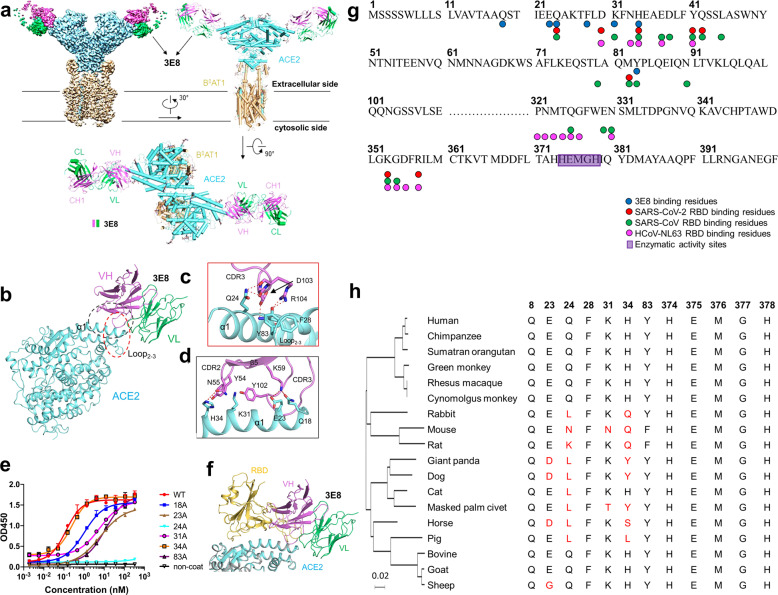
Fig. 6. Cryo-EM structure of the 3E8/ACE2-B0AT1 complex and “alanine walk” studies to solve the critical interactions between 3E8 and ACE2.
Domain-colored cryo-EM map (a, upper left panel) and the side view (a, upper right panel) or the top view (a, lower panel) of the structure. The heavy and light chains of 3E8 are colored green and violet, respectively. The ACE2 and B0AT1 are colored cyan and wheat, respectively. b Binding interface between 3E8 and ACE2, which contains two clusters that are labeled with red and black dashed ellipses and detailed shown in c and d, respectively. H-bonds are indicated by red dashed lines. Q (Gln) 24 and H (His)34 on ACE2 were identified as critical amino acid residues interacting with the CDR3 and CDR2 of heavy chain of 3E8, respectively. e “Alanine walk” identified Q24 on ACE2 as the most critical amino acid residue for 3E8 binding. f Structural alignment of the 3E8/ACE2-B0AT1 complex and the RBD/ACE2-B0AT1 complex (PDB ID: 6M17) shows clash between 3E8 and RBD of the SARS-CoV-2 S protein. g Summary of binding site of multiple coronaviruses on human ACE2. h Evolutionary tree of 3E8 binding site on ACE2 with different species